Electron Transport Chain Final Electron Acceptor
Muz Play
Mar 28, 2025 · 5 min read
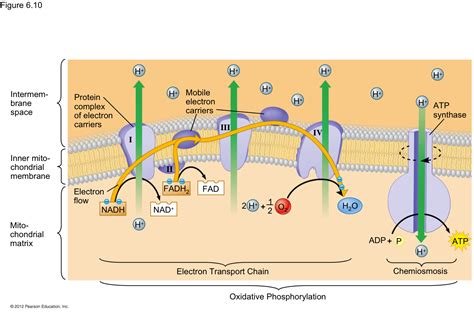
Table of Contents
The Electron Transport Chain's Final Electron Acceptor: Oxygen and Beyond
The electron transport chain (ETC), a cornerstone of cellular respiration, is a remarkable series of redox reactions that harvests energy from electrons. This energy is then used to generate a proton gradient, which drives the synthesis of ATP, the cell's primary energy currency. Crucial to the function of the ETC is the final electron acceptor, a molecule that receives the electrons at the end of the chain, completing the process. While oxygen is the most common final electron acceptor in aerobic organisms, other molecules can fulfill this role under anaerobic conditions. This article delves deep into the role of the final electron acceptor, focusing on oxygen's importance and exploring alternative acceptors in various organisms and environments.
The Role of Oxygen in Aerobic Respiration
In aerobic organisms, oxygen (O₂) serves as the terminal electron acceptor in the ETC. This is the defining characteristic of aerobic respiration, a highly efficient process that yields a significant amount of ATP. The reduction of oxygen is a crucial step because it allows the ETC to continue functioning. Without a final electron acceptor to receive the electrons, the electron transport chain would become blocked, halting ATP production.
The Reduction of Oxygen
The final step in the electron transport chain involves the transfer of electrons to oxygen, reducing it to water (H₂O). This reaction is catalyzed by cytochrome c oxidase, the terminal enzyme complex in the ETC. The reaction can be represented as follows:
4e⁻ + 4H⁺ + O₂ → 2H₂O
This reaction is energetically favorable, ensuring that the electrons flow continuously through the chain. The high reduction potential of oxygen makes it an excellent electron acceptor, driving the entire process. The formation of water is not just a byproduct; it’s a crucial component of the system's efficiency. The removal of electrons from the chain prevents the build-up of reduced electron carriers, maintaining the redox potential gradient that powers ATP synthesis.
Consequences of Oxygen Deficiency
The absence of oxygen dramatically alters the energy production pathway. Without oxygen as the final electron acceptor, the ETC becomes stalled, significantly reducing ATP production. This leads to a shift towards anaerobic respiration or fermentation, much less efficient energy-generating processes. The consequences can be severe, ranging from reduced cellular function to cell death, depending on the organism and the duration of oxygen deprivation.
Alternative Final Electron Acceptors: Anaerobic Respiration
While oxygen is the most common final electron acceptor, many organisms thrive in anaerobic environments, utilizing alternative electron acceptors in a process known as anaerobic respiration. These alternative acceptors have lower reduction potentials than oxygen, resulting in less ATP production compared to aerobic respiration. However, they allow these organisms to survive and thrive in oxygen-depleted environments.
Nitrate (NO₃⁻) Reduction
Nitrate is a common alternative electron acceptor used by many bacteria and archaea. The reduction of nitrate (NO₃⁻) to nitrite (NO₂⁻) is the first step in the process, catalyzed by the enzyme nitrate reductase. Further reduction can lead to the formation of nitric oxide (NO), nitrous oxide (N₂O), and finally, nitrogen gas (N₂). This process, known as denitrification, plays a significant role in the nitrogen cycle, converting nitrogen compounds back into atmospheric nitrogen.
Sulfate (SO₄²⁻) Reduction
Sulfate-reducing bacteria utilize sulfate (SO₄²⁻) as a terminal electron acceptor. The reduction of sulfate to hydrogen sulfide (H₂S) is a crucial process in anaerobic environments, such as sediments and marshes. This process is ecologically significant, contributing to the sulfur cycle and influencing the overall geochemical environment.
Carbon Dioxide (CO₂) Reduction
Some archaea, known as methanogens, use carbon dioxide (CO₂) as the final electron acceptor, reducing it to methane (CH₄). This process, called methanogenesis, is an important step in the carbon cycle and contributes significantly to methane emissions in various ecosystems, including wetlands and landfills.
Iron (Fe³⁺) Reduction
Iron-reducing bacteria can use ferric iron (Fe³⁺) as a terminal electron acceptor, reducing it to ferrous iron (Fe²⁺). This process is particularly prevalent in anoxic sediments and groundwater, playing a role in iron cycling and influencing the availability of iron for other organisms.
The Impact of Final Electron Acceptor on ATP Production
The choice of the final electron acceptor significantly impacts the amount of ATP produced during cellular respiration. Oxygen, with its high reduction potential, allows for a large proton gradient to be generated, resulting in the highest ATP yield. Alternative electron acceptors, having lower reduction potentials, lead to smaller proton gradients and consequently, lower ATP production. This difference in ATP yield highlights the importance of oxygen as the preferred terminal electron acceptor for efficient energy production.
The Evolutionary Significance of Different Final Electron Acceptors
The use of different final electron acceptors reflects the evolutionary adaptation of organisms to diverse environmental conditions. The prevalence of oxygen in the Earth's atmosphere favored the evolution of aerobic respiration, while the development of anaerobic respiration enabled organisms to exploit environments where oxygen is scarce or absent. The diversity of final electron acceptors demonstrates the remarkable adaptability of life on Earth.
Conclusion: A Diverse and Essential Process
The final electron acceptor in the electron transport chain is a critical component of cellular respiration, significantly impacting ATP production and the ecological roles of organisms. While oxygen is the most efficient and widely used final electron acceptor in aerobic respiration, the ability of various organisms to utilize alternative acceptors showcases the versatility and resilience of life on Earth. Understanding the mechanisms of different electron acceptors is vital for comprehending various ecological processes, from nutrient cycling to greenhouse gas emissions. Further research into this area could unlock new insights into the evolution of life and potentially lead to advancements in biotechnology and environmental remediation.
Further Exploration:
- The role of quinones in electron transport.
- The mechanisms of proton pumping in the ETC complexes.
- The regulation of electron transport chain activity.
- The impact of environmental factors on the selection of electron acceptors.
- The biotechnological applications of anaerobic respiration.
This in-depth exploration of the final electron acceptor in the electron transport chain provides a solid foundation for understanding this critical process in cellular respiration. The diverse array of electron acceptors highlights the remarkable adaptability of life and the intricate interplay between organisms and their environment. The field continues to expand with ongoing research exploring the intricacies of electron transport and its role in various ecosystems.
Latest Posts
Latest Posts
-
How To Write Compounds In Chemistry
Mar 31, 2025
-
Identify The Components Of Energy Output Not Involving Basal Metabolism
Mar 31, 2025
-
Boiling Water Is A Chemical Change
Mar 31, 2025
-
Does Plasma Have A Definite Shape
Mar 31, 2025
-
Using The Small X Approximation To Solve Equilibrium Problems
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Electron Transport Chain Final Electron Acceptor . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
