Electrons In A Polar Covalent Bond Are Shared Equally
Muz Play
Mar 18, 2025 · 6 min read
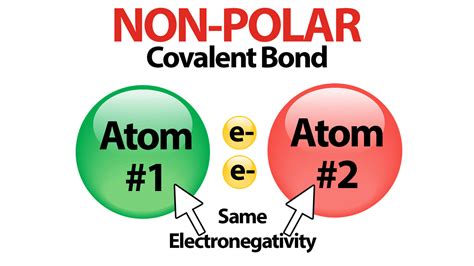
Table of Contents
Do Electrons in a Polar Covalent Bond Share Equally? A Deep Dive into Electronegativity and Bond Polarity
The statement "electrons in a polar covalent bond are shared equally" is fundamentally incorrect. This misconception often arises from a blurry understanding of the differences between covalent, polar covalent, and ionic bonds. While all three involve the interaction of electrons between atoms, the degree of electron sharing drastically differs, leading to varying bond polarities. This article will delve deep into the nature of covalent bonds, exploring the nuances of electron sharing and explaining why polar covalent bonds, by definition, involve unequal electron sharing.
Understanding Covalent Bonds: A Shared Resource
Covalent bonds are formed when two atoms share electrons to achieve a more stable electron configuration, typically resembling a noble gas. This sharing occurs because both atoms involved have relatively similar electronegativities (more on that later). The shared electrons occupy molecular orbitals, regions of space where the probability of finding the electrons is high.
Examples of Covalent Bonds:
- Hydrogen gas (H₂): Two hydrogen atoms share a pair of electrons, each contributing one electron to the shared pair. This results in a stable duet for each hydrogen atom.
- Methane (CH₄): A carbon atom shares four electron pairs, one with each of four hydrogen atoms, fulfilling the octet rule for carbon and duet rule for each hydrogen.
- Oxygen gas (O₂): Two oxygen atoms share two pairs of electrons, forming a double bond and achieving a stable octet for each oxygen atom.
Electronegativity: The Driving Force Behind Bond Polarity
Electronegativity is a crucial concept in understanding bond polarity. It's a measure of an atom's ability to attract electrons towards itself within a chemical bond. Atoms with high electronegativity strongly attract electrons, while atoms with low electronegativity hold electrons less tightly. The Pauling scale is commonly used to represent electronegativity values, with fluorine (F) having the highest value (4.0).
The Role of Electronegativity Difference:
The difference in electronegativity between two atoms determines the nature of the bond formed:
-
Nonpolar Covalent Bond: When the electronegativity difference between two atoms is very small (generally less than 0.5 on the Pauling scale), the electrons are shared relatively equally between them. This results in a nonpolar covalent bond with no significant charge separation.
-
Polar Covalent Bond: When the electronegativity difference is moderate (generally between 0.5 and 1.7 on the Pauling scale), the electrons are shared unequally. The atom with higher electronegativity attracts the electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. This unequal sharing of electrons leads to a polar covalent bond with a dipole moment (a measure of the bond's polarity).
-
Ionic Bond: When the electronegativity difference is large (generally greater than 1.7 on the Pauling scale), the more electronegative atom essentially steals an electron from the less electronegative atom. This results in the formation of ions (charged atoms or molecules) and an ionic bond, characterized by a complete transfer of electrons.
Why "Equal Sharing" is Wrong for Polar Covalent Bonds
The core misunderstanding in the initial statement lies in the definition of a polar covalent bond. By definition, a polar covalent bond involves unequal sharing of electrons. If the electrons were shared equally, the bond would be nonpolar. The presence of a dipole moment, a clear indication of charge separation, distinguishes a polar covalent bond from its nonpolar counterpart.
Examples Illustrating Unequal Sharing:
-
Water (H₂O): Oxygen is significantly more electronegative than hydrogen. In the O-H bonds of water, the electrons are pulled more towards the oxygen atom, giving oxygen a partial negative charge (δ-) and hydrogen a partial positive charge (δ+). This unequal sharing creates a polar covalent bond and makes water a polar molecule.
-
Hydrogen Chloride (HCl): Chlorine is more electronegative than hydrogen. The electrons in the H-Cl bond are drawn more towards chlorine, resulting in a partial negative charge (δ-) on chlorine and a partial positive charge (δ+) on hydrogen.
-
Ammonia (NH₃): Nitrogen is more electronegative than hydrogen, leading to unequal electron sharing in the N-H bonds. The nitrogen atom carries a partial negative charge, while the hydrogen atoms carry partial positive charges.
The Impact of Unequal Electron Sharing: Properties of Polar Molecules
The unequal sharing of electrons in polar covalent bonds has significant consequences for the properties of the resulting molecules. These properties include:
-
Higher boiling and melting points: The dipole-dipole interactions between polar molecules are stronger than the weaker London dispersion forces present in nonpolar molecules. These stronger interactions require more energy to overcome, leading to higher boiling and melting points.
-
Solubility in polar solvents: Polar molecules are generally soluble in polar solvents (like water) because of the strong dipole-dipole interactions between them. "Like dissolves like" is a common rule of thumb in chemistry.
-
Electrical conductivity: While not conducting electricity in the same way as ionic compounds, polar molecules can exhibit some dielectric properties, allowing them to interact with electric fields.
-
Reactivity: The partial charges on polar molecules make them more reactive than their nonpolar counterparts. The polarized regions can attract other charged species or participate in chemical reactions more readily.
Delving Deeper: Factors Influencing Electronegativity
Several factors influence an atom's electronegativity:
-
Nuclear Charge: A higher nuclear charge leads to a stronger attraction for electrons.
-
Atomic Radius: Smaller atoms have a greater electronegativity because the electrons are closer to the nucleus, experiencing stronger attraction.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus, reducing the electronegativity.
-
Electron Configuration: The stability of the electron configuration also influences electronegativity. Atoms closer to achieving a noble gas configuration generally exhibit higher electronegativity.
Beyond the Basics: Advanced Concepts in Bond Polarity
While the electronegativity difference provides a good initial estimate of bond polarity, more sophisticated methods exist for determining the precise degree of electron sharing and the resulting dipole moment. These methods often involve computational chemistry and quantum mechanical calculations. These calculations provide a more accurate picture of electron distribution within molecules and can be particularly important for complex molecules with multiple polar bonds.
Furthermore, the concept of resonance in molecules with delocalized electrons (like benzene) adds further complexity to determining bond polarity. The electrons are not confined to individual bonds but rather are spread over multiple atoms, leading to a more nuanced description of charge distribution.
Conclusion: Understanding the Nuances of Covalent Bonding
The statement that electrons in a polar covalent bond are shared equally is inaccurate. Polar covalent bonds, by their very nature, involve unequal sharing of electrons driven by the difference in electronegativity between the bonded atoms. This unequal sharing leads to a dipole moment and significantly influences the physical and chemical properties of the resulting molecules. Understanding the nuances of electronegativity and its role in determining bond polarity is essential for a comprehensive grasp of chemical bonding and molecular behavior. The difference between nonpolar and polar covalent bonds is not just a minor detail; it's a fundamental distinction that determines many key properties and reactivity patterns in the vast world of chemistry.
Latest Posts
Latest Posts
-
Examples Of Modified Stem Of A Plant
Mar 19, 2025
-
During Glycolysis Glucose Is Broken Down Into
Mar 19, 2025
-
How To Identify An Acid From Its Chemical Formula
Mar 19, 2025
-
Investigation Dna Proteins And Sickle Cell Answer Key
Mar 19, 2025
-
Buffer Region On A Titration Curve
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Electrons In A Polar Covalent Bond Are Shared Equally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
