How To Identify An Acid From Its Chemical Formula
Muz Play
Mar 19, 2025 · 7 min read
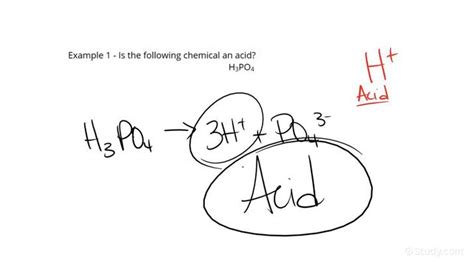
Table of Contents
How to Identify an Acid from its Chemical Formula
Identifying acids solely from their chemical formulas might seem daunting at first, but with a systematic approach and understanding of key principles, it becomes a straightforward process. This comprehensive guide will equip you with the knowledge and techniques to confidently classify chemical formulas as acids or not. We'll explore different types of acids, their defining characteristics, and practical examples to solidify your understanding.
Understanding Acids: The Fundamental Concepts
Before delving into formula identification, let's establish a firm foundation in acid chemistry. Acids are substances that donate protons (H⁺ ions) when dissolved in water. This proton donation is the defining characteristic of an acid, leading to an increase in the concentration of hydronium ions (H₃O⁺) in the solution. This increase in H₃O⁺ lowers the pH, making the solution acidic. The lower the pH, the stronger the acid.
There are two main categories of acids:
1. Binary Acids
Binary acids contain only two elements: hydrogen and a nonmetal. The general formula for a binary acid is HX, where H represents hydrogen and X represents a nonmetal. Examples include:
- Hydrochloric acid (HCl): A strong acid commonly found in gastric juices and used in industrial cleaning.
- Hydrobromic acid (HBr): Another strong acid used in various chemical processes.
- Hydroiodic acid (HI): Also a strong acid with similar applications to HBr.
- Hydrofluoric acid (HF): A relatively weak acid, despite being a binary acid. This is due to the strong hydrogen-fluorine bond.
Identifying binary acids from their formula is straightforward: Look for a formula starting with "H" followed by a single nonmetal.
2. Ternary (Oxyacids) Acids
Ternary acids, also known as oxyacids, contain hydrogen, a nonmetal, and oxygen. They're more complex than binary acids and exhibit diverse strengths. The general formula is often represented as HₓYOₙ, where H represents hydrogen, Y represents a nonmetal (often a central atom), and O represents oxygen. The subscript 'x' and 'n' vary depending on the specific acid. Examples include:
- Sulfuric acid (H₂SO₄): A very strong diprotic acid (donates two protons) widely used in industrial processes.
- Nitric acid (HNO₃): A strong monoprotic acid (donates one proton) used in fertilizer production and other applications.
- Phosphoric acid (H₃PO₄): A weaker triprotic acid (donates three protons) used in fertilizers and food additives.
- Carbonic acid (H₂CO₃): A weak diprotic acid found in carbonated beverages and responsible for the acidity in rain.
- Acetic acid (CH₃COOH): A weak monoprotic organic acid commonly found in vinegar. Note that while it follows a different structural representation, it still donates one proton, thus functioning as an acid. Organic acids are a subset of ternary acids and often contain carbon and hydrogen in addition to the other elements.
Identifying ternary acids requires a more detailed analysis: Look for a formula containing hydrogen (H), a nonmetal other than H, and oxygen (O). The nonmetal is often centrally located in the Lewis structure.
Steps to Identify an Acid from its Chemical Formula
Let's break down the identification process into clear, actionable steps:
-
Identify the presence of Hydrogen (H): The first and most crucial step is to check if the chemical formula begins with or contains hydrogen (H). All acids contain at least one hydrogen atom that can be donated as a proton. If hydrogen is absent, the compound is almost certainly not an acid.
-
Identify the other elements: After confirming the presence of hydrogen, examine the other elements present in the formula. Are they all nonmetals? If so, proceed to the next step. If you see a metal, the compound is likely an ionic compound and not an acid.
-
Classify as binary or ternary: Based on the number of elements present, classify the compound.
- Two elements (H and a nonmetal): This indicates a binary acid. The naming convention often includes "hydro-" followed by the nonmetal's root name with the "-ic" suffix and "acid". (e.g., HCl – hydrochloric acid).
- Three or more elements (H, a nonmetal, and oxygen): This suggests a ternary acid (or oxyacid). The naming convention is more complex, depending on the oxidation state of the central nonmetal.
-
Consider the oxidation state (for ternary acids): For ternary acids, the oxidation state of the central nonmetal influences the acid's name and strength. Higher oxidation states generally result in stronger acids. For example, nitric acid (HNO₃) with nitrogen in a +5 oxidation state is stronger than nitrous acid (HNO₂) with nitrogen in a +3 oxidation state.
-
Recognize exceptions: While the rules are generally reliable, some exceptions exist. Some compounds containing hydrogen may not behave as acids under all conditions. The context – including the solvent and the presence of other reactants – can influence whether a compound acts as an acid. For example, some organic compounds may contain hydrogen atoms but do not readily donate protons.
Practical Examples: Identifying Acids
Let's solidify your understanding with several examples:
Example 1: H₂SO₄ (Sulfuric Acid)
- Hydrogen present: Yes.
- Other elements: Sulfur (S) and Oxygen (O) – all nonmetals.
- Classification: Ternary acid (oxyacid).
- Conclusion: H₂SO₄ is an acid.
Example 2: HNO₃ (Nitric Acid)
- Hydrogen present: Yes.
- Other elements: Nitrogen (N) and Oxygen (O) – all nonmetals.
- Classification: Ternary acid (oxyacid).
- Conclusion: HNO₃ is an acid.
Example 3: HCl (Hydrochloric Acid)
- Hydrogen present: Yes.
- Other elements: Chlorine (Cl) – a nonmetal.
- Classification: Binary acid.
- Conclusion: HCl is an acid.
Example 4: NaOH (Sodium Hydroxide)
- Hydrogen present: Yes, but hydrogen is bonded to a metal, indicating it is not readily donatable as a proton.
- Other elements: Sodium (Na), a metal, is present.
- Classification: This is a strong base, not an acid. While it does contain hydrogen, its behavior in solution is completely different from that of an acid.
- Conclusion: NaOH is not an acid.
Example 5: CH₄ (Methane)
- Hydrogen present: Yes, but hydrogen is bonded to carbon, indicating a non-polar covalent bond and making it difficult to dissociate H⁺ ions in water.
- Other elements: Carbon (C) is a nonmetal. However, this is an organic compound and does not readily donate protons to behave as an acid under normal circumstances.
- Classification: Organic compound; not an acid.
- Conclusion: CH₄ is not an acid in typical chemical reactions.
Advanced Considerations: Amphoteric Substances and Lewis Acids
The Brønsted-Lowry definition of acids (proton donors) is widely used, but it doesn't encompass all acidic behavior.
-
Amphoteric Substances: Some substances can act as both acids and bases, depending on the chemical environment. Water (H₂O) is a classic example. It can act as an acid by donating a proton or a base by accepting a proton. Identifying amphoteric substances from their formula requires considering the potential for both proton donation and acceptance.
-
Lewis Acids: The Lewis definition of an acid broadens the concept to include electron-pair acceptors. Lewis acids don't necessarily contain hydrogen. For instance, boron trifluoride (BF₃) is a Lewis acid because boron can accept an electron pair. Identifying Lewis acids solely from the formula requires understanding the electronic structure and the ability of the central atom to accept an electron pair. This is significantly more complex than the Brønsted-Lowry approach and typically requires deeper knowledge of chemical bonding and electronic configurations.
Conclusion: Mastering Acid Identification
Identifying acids from chemical formulas is a fundamental skill in chemistry. By systematically analyzing the elements present, classifying the compound as binary or ternary, and considering the oxidation states (for ternary acids), you can confidently determine whether a given formula represents an acid. Remember to be mindful of exceptions and advanced concepts like amphoteric substances and Lewis acids for a comprehensive understanding. Practice is key to mastering this skill, and by working through various examples, you'll enhance your ability to rapidly and accurately identify acids from their chemical formulas. This understanding forms the bedrock of numerous chemical concepts and reactions.
Latest Posts
Latest Posts
-
Is Salt Water A Substance Or Mixture
Mar 19, 2025
-
Where Are The Focus Controls On A Microscope Located
Mar 19, 2025
-
Non Homogeneous First Order Differential Equation
Mar 19, 2025
-
Using Inverse Matrix To Solve System Of Linear Equations
Mar 19, 2025
-
Is H2o An Acid Or Base
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Identify An Acid From Its Chemical Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
