Element Families Of The Periodic Table
Muz Play
Mar 23, 2025 · 7 min read
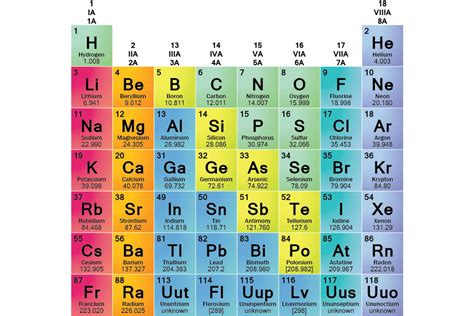
Table of Contents
Delving Deep into the Element Families of the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements not haphazardly, but according to their properties and electron configurations. This organization reveals striking patterns, grouping elements into families or groups that share similar chemical behaviors. Understanding these families is key to grasping the fundamental principles governing chemical reactions and the properties of matter. This article will explore the major element families, delving into their characteristics, trends, and significance.
The Alkali Metals (Group 1)
The alkali metals, located in the first column of the periodic table, are highly reactive metals. This reactivity stems from their electronic structure; they possess only one electron in their outermost shell (valence shell), readily losing it to achieve a stable octet. This single valence electron is easily ionized, leading to the formation of +1 ions.
Key Characteristics:
- Highly Reactive: Their extreme reactivity makes them readily react with water, air, and other elements. Reactions with water are often vigorous, producing hydrogen gas and a metal hydroxide.
- Low Density: They are significantly less dense than other metals, with lithium, sodium, and potassium being lighter than water.
- Low Melting and Boiling Points: Compared to other metals, they have relatively low melting and boiling points.
- Good Electrical and Thermal Conductors: Their loosely held valence electrons contribute to their excellent conductivity.
- Soft and Easily Cut: They are soft enough to be cut with a knife.
Notable Members and Applications:
- Lithium (Li): Used in batteries, ceramics, and lubricants. Its isotope, lithium-6, has applications in nuclear fusion.
- Sodium (Na): Essential for human life (sodium chloride – table salt), used in streetlights (sodium-vapor lamps), and in the production of various chemicals.
- Potassium (K): Crucial for plant growth and essential for human bodily functions. Used in fertilizers and some types of glass.
- Rubidium (Rb) and Cesium (Cs): Less common but have applications in atomic clocks and specific scientific instruments. Cesium is used as a standard in atomic clocks because of its precise atomic transitions.
The Alkaline Earth Metals (Group 2)
The alkaline earth metals, residing in the second column, are also reactive metals, though less so than the alkali metals. They possess two valence electrons, which they readily lose to form +2 ions.
Key Characteristics:
- Reactive (less than Alkali Metals): While reactive, their reactivity is lower than the alkali metals. They still react with water, though often more slowly.
- Higher Density than Alkali Metals: They possess higher density than their alkali metal counterparts.
- Higher Melting and Boiling Points than Alkali Metals: Their melting and boiling points are generally higher than those of the alkali metals.
- Good Electrical and Thermal Conductors: Similar to alkali metals, they are good conductors of electricity and heat.
- Relatively Hard: They are harder and denser than alkali metals.
Notable Members and Applications:
- Beryllium (Be): Used in aerospace alloys due to its high strength-to-weight ratio. It's also used in X-ray windows.
- Magnesium (Mg): Lightweight and strong, used in alloys for airplanes, automobiles, and other applications. It is also an important element in chlorophyll.
- Calcium (Ca): Essential for bone formation in animals, used in cement, and in various metallurgical processes.
- Strontium (Sr) and Barium (Ba): Used in fireworks (Strontium for red, Barium for green) and in specific types of glass.
The Halogens (Group 17)
The halogens, found in Group 17, are highly reactive nonmetals. They are characterized by having seven valence electrons, readily gaining one electron to achieve a stable octet and form -1 ions (anions).
Key Characteristics:
- Highly Reactive Nonmetals: Their high reactivity is due to their strong tendency to gain an electron.
- Diatomic Molecules: They exist as diatomic molecules (e.g., Cl₂, Br₂) in their elemental form.
- Varied Physical States: They exhibit different physical states at room temperature: fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid.
- Oxidizing Agents: They act as strong oxidizing agents, readily accepting electrons from other substances.
- Colored Vapors: Many halogens produce distinctive colored vapors.
Notable Members and Applications:
- Fluorine (F): Used in toothpaste (fluoride) to prevent tooth decay, and in the production of various fluorocarbons.
- Chlorine (Cl): Used in water purification (chlorination), as a bleaching agent, and in the production of various chemicals, including PVC.
- Bromine (Br): Used as a flame retardant, in photography, and in certain dyes.
- Iodine (I): Essential for thyroid function in humans, used as an antiseptic, and in certain dyes.
- Astatine (At): A radioactive element with limited applications, primarily in research.
The Noble Gases (Group 18)
The noble gases, located in Group 18, are unique in their chemical inertness. They possess a complete octet of electrons in their valence shell, making them extremely stable and unreactive. They rarely form compounds.
Key Characteristics:
- Inert Gases: Their stable electron configuration makes them exceptionally unreactive.
- Monoatomic Gases: They exist as monatomic gases under normal conditions.
- Colorless, Odorless, and Tasteless: They are generally colorless, odorless, and tasteless.
- Low Boiling Points: They have very low boiling points.
- Used in Lighting: Their inertness and ability to emit light when excited make them useful in lighting applications (neon lights, etc.).
Notable Members and Applications:
- Helium (He): Used in balloons, cryogenics, and MRI machines. It's the second most abundant element in the universe.
- Neon (Ne): Used in neon signs.
- Argon (Ar): Used as an inert atmosphere in welding and other processes where reactivity needs to be prevented.
- Krypton (Kr): Used in some lasers and photographic flash lamps.
- Xenon (Xe): Used in some high-intensity lamps and in medical imaging.
- Radon (Rn): A radioactive gas, considered a health hazard.
Transition Metals (Groups 3-12)
The transition metals occupy the central region of the periodic table. They are characterized by their partially filled d orbitals, leading to a variety of oxidation states and complex ion formation. This results in a wide range of chemical and physical properties.
Key Characteristics:
- Variable Oxidation States: They can exist in multiple oxidation states, leading to diverse chemical behaviors.
- Formation of Colored Compounds: Many transition metal compounds are brightly colored.
- Catalytic Activity: Many transition metals and their compounds exhibit catalytic activity, speeding up chemical reactions.
- Magnetic Properties: Some transition metals exhibit magnetic properties (ferromagnetism, paramagnetism).
- High Melting and Boiling Points: Generally, they have high melting and boiling points.
Notable Members and Applications:
- Iron (Fe): Essential for oxygen transport in blood (hemoglobin), used in steel production, and various other applications.
- Copper (Cu): Excellent conductor of electricity, used in electrical wiring, plumbing, and alloys (brass, bronze).
- Zinc (Zn): Used in galvanization (corrosion protection), in batteries, and various alloys.
- Gold (Au) and Silver (Ag): Precious metals valued for their inertness, conductivity, and beauty, used in jewelry, electronics, and currency.
- Platinum (Pt): A valuable catalyst used in various industrial processes and in automotive catalytic converters.
Lanthanides and Actinides (f-block elements)
The lanthanides (rare earth elements) and actinides are placed below the main body of the periodic table. They are characterized by the filling of the 4f and 5f orbitals, respectively. Many are radioactive.
Key Characteristics:
- Similar Chemical Properties: Lanthanides exhibit very similar chemical properties due to the shielding effect of the 4f electrons.
- Radioactivity: Many actinides are radioactive.
- Applications in Specialized Technologies: Used in various high-tech applications like lasers, magnets, and nuclear reactors.
Notable Members and Applications:
- Lanthanum (La): Used in some alloys and in lighting applications.
- Cerium (Ce): Used in self-cleaning ovens and as a catalyst.
- Uranium (U) and Plutonium (Pu): Used in nuclear reactors and weapons.
Understanding Periodic Trends
The arrangement of the elements in the periodic table allows us to predict trends in various properties. These trends are crucial for understanding chemical reactivity and behavior. Key periodic trends include:
- Atomic Radius: Generally increases down a group and decreases across a period.
- Ionization Energy: Generally decreases down a group and increases across a period.
- Electronegativity: Generally decreases down a group and increases across a period.
- Electron Affinity: Shows a more complex trend, influenced by electron configuration.
Conclusion
The periodic table, with its organization into element families, is an indispensable tool for chemists and scientists. Understanding the properties and trends within these families provides a fundamental framework for comprehending chemical reactions, predicting the behavior of substances, and developing new materials and technologies. The exploration of these element families reveals the intricate interplay of atomic structure and chemical properties, highlighting the elegance and power of the periodic system. Further study into specific elements and their applications within each family will only deepen your understanding of the remarkable world of chemistry.
Latest Posts
Latest Posts
-
How To Calculate Total Magnification On A Microscope
Mar 24, 2025
-
How To Find The Frequency Of Oscillation
Mar 24, 2025
-
A Perfectly Elastic Supply Curve Is
Mar 24, 2025
-
Bones Of The Pectoral Girdle And Upper Limb
Mar 24, 2025
-
People Forsaken By Their Own God
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Element Families Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
