Elements In Compounds Are Held Together By Chemical
Muz Play
Mar 16, 2025 · 7 min read
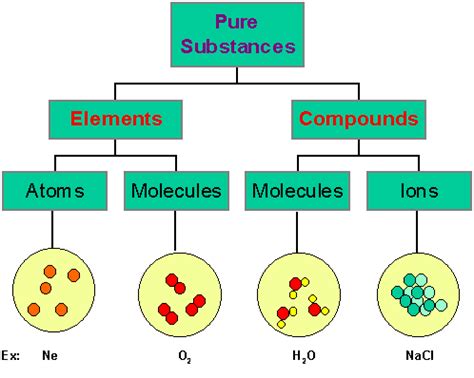
Table of Contents
Elements in Compounds are Held Together by Chemical Bonds: A Deep Dive
The world around us is composed of matter, and matter, at its most fundamental level, consists of elements. However, rarely do we encounter elements in their pure, uncombined form. Instead, elements typically combine to form compounds, held together by the powerful forces of chemical bonds. Understanding these bonds is crucial to understanding the properties and behavior of all matter, from the simplest molecules to the most complex biological systems. This article will explore the various types of chemical bonds, their formation, and the properties they impart to compounds.
The Nature of Chemical Bonding
Chemical bonds are essentially the attractive forces that hold atoms together in compounds. These forces arise from the electrostatic interactions between the positively charged nuclei and the negatively charged electrons of the atoms involved. The driving force behind bond formation is the achievement of a more stable electronic configuration, usually resembling that of a noble gas (a group 18 element with a full valence electron shell). This stability translates to lower potential energy, making the bonded state more favorable than the individual, unbonded atoms.
There are several primary types of chemical bonds:
1. Ionic Bonds: The Electrostatic Attraction
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal with low electronegativity (a measure of an atom's ability to attract electrons), loses one or more electrons to become a positively charged cation. Simultaneously, another atom, usually a nonmetal with high electronegativity, gains these electrons to become a negatively charged anion. The resulting electrostatic attraction between the cation and anion forms the ionic bond.
Example: Consider the formation of sodium chloride (NaCl), common table salt. Sodium (Na), a metal, readily loses one electron to achieve a stable electron configuration, becoming a Na⁺ ion. Chlorine (Cl), a nonmetal, readily gains this electron to achieve a stable configuration, becoming a Cl⁻ ion. The strong electrostatic attraction between Na⁺ and Cl⁻ ions forms the ionic bond, resulting in a crystalline lattice structure of NaCl.
Properties of Ionic Compounds: Ionic compounds typically exhibit high melting and boiling points due to the strong electrostatic forces holding the ions together. They are often brittle and tend to dissolve readily in polar solvents like water, as the polar water molecules can effectively interact with and separate the ions. In their solid state, they are usually poor conductors of electricity, but they become good conductors when molten or dissolved in water, as the ions become mobile.
2. Covalent Bonds: Sharing is Caring
Covalent bonds involve the sharing of electrons between two atoms. This occurs most often between nonmetal atoms with similar electronegativities. Instead of transferring electrons completely, as in ionic bonding, atoms share electrons to achieve a stable electron configuration. The shared electrons are attracted to the nuclei of both atoms, creating a bond that holds the atoms together.
Example: Consider the formation of a water molecule (H₂O). Each hydrogen atom shares one electron with the oxygen atom, and the oxygen atom shares one electron with each hydrogen atom. This sharing of electrons results in two covalent bonds, forming a stable water molecule.
Types of Covalent Bonds:
-
Nonpolar Covalent Bonds: These bonds occur when the electronegativity difference between the atoms is very small or zero. The electrons are shared equally between the atoms. Examples include bonds within diatomic molecules like H₂, O₂, and N₂.
-
Polar Covalent Bonds: These bonds occur when there is a significant difference in electronegativity between the atoms. The electrons are shared unequally, resulting in a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. The water molecule (H₂O) provides a good example of a molecule with polar covalent bonds.
Properties of Covalent Compounds: Covalent compounds generally have lower melting and boiling points compared to ionic compounds because the covalent bonds are weaker than ionic bonds. They can exist as solids, liquids, or gases at room temperature, depending on the strength of the intermolecular forces. They are often poor conductors of electricity in both solid and liquid states because they lack freely moving charged particles.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals and alloys. In metals, the valence electrons are delocalized, meaning they are not associated with any particular atom but are free to move throughout the entire metal structure. This creates a "sea" of electrons surrounding a lattice of positively charged metal ions. The electrostatic attraction between the positively charged ions and the delocalized electrons holds the metal together.
Properties of Metals: The delocalized electrons account for many characteristic properties of metals, such as their excellent electrical and thermal conductivity (due to the mobility of electrons), their malleability (ability to be hammered into shapes), and their ductility (ability to be drawn into wires).
4. Hydrogen Bonds: Special Interactions
Hydrogen bonds are a special type of intermolecular force, not a true chemical bond. They occur between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. The highly electronegative atom pulls the electron density away from the hydrogen atom, creating a partial positive charge (δ+) on the hydrogen. This partially positive hydrogen is then attracted to the partially negative (δ-) region of another electronegative atom in a nearby molecule.
Example: Water molecules are held together by hydrogen bonds. The partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another water molecule.
Importance of Hydrogen Bonds: Hydrogen bonds play crucial roles in many biological systems, including the structure of proteins and DNA. They are responsible for the relatively high boiling point of water compared to other similar molecules.
Factors Affecting Bond Strength
The strength of a chemical bond is determined by several factors:
-
Electronegativity Difference: The larger the electronegativity difference between atoms involved in a bond, the stronger the bond (particularly in ionic and polar covalent bonds).
-
Bond Order: Bond order refers to the number of electron pairs shared between two atoms. A higher bond order generally indicates a stronger bond. For instance, a triple bond (like in N₂) is stronger than a double bond (like in O₂) which, in turn, is stronger than a single bond (like in H₂).
-
Atomic Size: Smaller atoms generally form stronger bonds because the nuclei are closer together, leading to stronger electrostatic attraction.
-
Bond Length: The distance between the nuclei of two bonded atoms is known as bond length. Shorter bond lengths generally correspond to stronger bonds.
Applications and Significance of Chemical Bonding
Understanding chemical bonds is essential in various fields:
-
Chemistry: Predicting the properties of compounds, designing new materials, and understanding chemical reactions all rely on a solid grasp of chemical bonding principles.
-
Materials Science: The design of new materials with specific properties, such as strength, conductivity, or reactivity, requires a deep understanding of how chemical bonds influence material behavior.
-
Biology: Chemical bonding is fundamental to understanding the structure and function of biological molecules like proteins, DNA, and carbohydrates. The intricate interactions between these molecules, governed by chemical bonds, are essential for life processes.
-
Medicine: Drug design and development often involve manipulating chemical bonds to create molecules with specific therapeutic effects. Understanding how drugs interact with biological targets at the molecular level is vital for effective treatment.
Conclusion
Chemical bonds are the fundamental forces holding atoms together in compounds. The different types of chemical bonds—ionic, covalent, metallic, and hydrogen bonds—lead to a wide range of properties in substances. A thorough understanding of chemical bonding is paramount for advancing knowledge and innovation across various scientific disciplines, ultimately shaping our world and improving our lives. Further exploration into the intricacies of bond energies, hybridization, and molecular geometry will offer even deeper insights into the fascinating world of chemical bonding.
Latest Posts
Latest Posts
-
Can You Be In Love With Two People
Mar 17, 2025
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Elements In Compounds Are Held Together By Chemical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
