Eqn For Charge In Electro Chemistry
Muz Play
Mar 15, 2025 · 6 min read
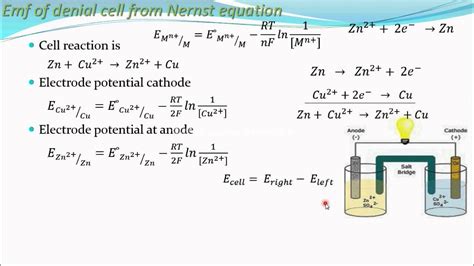
Table of Contents
The Equations Governing Charge in Electrochemistry: A Comprehensive Guide
Electrochemistry, the study of the relationship between chemical reactions and electrical energy, relies heavily on a set of fundamental equations to describe the behavior of charged species. Understanding these equations is crucial for comprehending a wide range of electrochemical phenomena, from battery operation to corrosion processes. This article provides a comprehensive overview of the key equations governing charge in electrochemistry, explaining their derivation, applications, and limitations.
Faraday's Laws of Electrolysis: The Foundation of Electrochemical Quantification
The cornerstone of quantitative electrochemistry is Faraday's Laws of Electrolysis. These laws connect the amount of substance produced or consumed in an electrochemical reaction to the quantity of electric charge passed through the system.
Faraday's First Law:
Faraday's First Law states that the amount of substance deposited or liberated at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. Mathematically, this is expressed as:
m = ZQ
Where:
- m is the mass of the substance (in grams)
- Z is the electrochemical equivalent (the mass of substance deposited or liberated per unit charge, expressed in grams per coulomb)
- Q is the quantity of electricity (in coulombs), given by Q = It, where I is the current (in amperes) and t is the time (in seconds).
This law highlights the fundamental relationship between charge and mass transfer in electrochemical processes. The more charge passed, the greater the amount of substance transformed.
Faraday's Second Law:
Faraday's Second Law states that when the same quantity of electricity is passed through solutions of different electrolytes, the masses of the substances deposited or liberated are proportional to their equivalent weights. The equivalent weight is the molar mass of the substance divided by the number of electrons transferred in the electrochemical reaction.
This law emphasizes the importance of the stoichiometry of the electrochemical reaction. Different reactions, even with the same charge passed, will produce different amounts of product depending on the number of electrons involved.
The Nernst Equation: Equilibrium Potentials and Concentration Dependence
The Nernst Equation is a pivotal equation in electrochemistry, linking the potential of an electrochemical cell to the concentrations of the reacting species. It's crucial for understanding how concentration gradients drive electrochemical reactions and for determining equilibrium potentials. The general form of the Nernst equation is:
E = E° - (RT/nF)lnQ
Where:
- E is the cell potential under non-standard conditions (in volts)
- E° is the standard cell potential (in volts) – the cell potential when all reactants and products are at unit activity (often approximated as 1 M concentration)
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature (in Kelvin)
- n is the number of moles of electrons transferred in the balanced redox reaction
- F is the Faraday constant (96485 C/mol)
- Q is the reaction quotient – the ratio of the activities (or concentrations) of products to reactants, each raised to the power of its stoichiometric coefficient.
The Nernst equation shows how the cell potential deviates from its standard value when the concentrations of reactants and products are not unity. It's essential for predicting the cell's behavior under various conditions, including those found in real-world electrochemical systems like batteries and fuel cells.
Applications of the Nernst Equation:
- Determining Equilibrium Constants: At equilibrium, E = 0, and the Nernst equation can be rearranged to solve for the equilibrium constant, K.
- Predicting Cell Potential under Non-Standard Conditions: The Nernst equation allows for the calculation of cell potential under various concentration and temperature conditions.
- Understanding the Role of Concentration Gradients: It demonstrates how concentration differences drive the electrochemical reaction and contribute to the cell potential.
The Butler-Volmer Equation: Kinetics of Electrode Reactions
While the Nernst equation describes equilibrium potentials, the Butler-Volmer equation addresses the kinetics of electrode reactions, focusing on the rate of electron transfer at the electrode-electrolyte interface. It describes the current density (i) as a function of the overpotential (η), which is the difference between the actual electrode potential and the equilibrium potential. The general form is:
i = i₀[exp(αnFη/RT) - exp(-(1-α)nFη/RT)]
Where:
- i is the current density (A/m²)
- i₀ is the exchange current density (A/m²) – a measure of the rate of electron transfer at equilibrium
- α is the charge transfer coefficient (dimensionless) – a parameter reflecting the symmetry of the energy barrier for electron transfer
- n, F, R, T, and η are as defined previously.
The Butler-Volmer equation is crucial for understanding how the rate of an electrochemical reaction depends on the applied potential and the intrinsic properties of the electrode-electrolyte interface. It forms the basis for analyzing various electrochemical techniques, such as voltammetry and chronoamperometry.
Significance of the Butler-Volmer Equation:
- Describing Electrochemical Kinetics: It provides a quantitative description of the relationship between the current and the overpotential.
- Analyzing Polarization Curves: It helps in interpreting polarization curves, which are plots of current density versus overpotential.
- Understanding the Effects of Overpotential: It demonstrates how overpotential influences the rate of electrochemical reactions.
Tafel Equation: High Overpotential Approximation of Butler-Volmer
At high overpotentials (|η| >> RT/nF), the Butler-Volmer equation simplifies to the Tafel equation:
η = a + b log|i|
Where:
- a and b are Tafel constants, related to the exchange current density and the charge transfer coefficient.
The Tafel equation is useful for analyzing electrochemical systems operating far from equilibrium, such as in corrosion or electroplating processes. The Tafel plot, a graph of η versus log|i|, provides valuable information about the electrochemical reaction kinetics and the nature of the electrode process.
Other Important Equations in Electrochemistry
Beyond the core equations discussed above, several other equations play crucial roles in electrochemistry. These include:
- Ohm's Law: V = IR, where V is the voltage, I is the current, and R is the resistance. This is fundamental to understanding the voltage drop across an electrochemical cell due to its internal resistance.
- Capacitance Equations: Electrochemical interfaces exhibit capacitive behavior. Equations describing capacitance are important for understanding the charging and discharging of the electric double layer at the electrode-electrolyte interface.
- Diffusion Equations: Mass transport by diffusion significantly influences electrochemical reactions. Fick's laws of diffusion are critical for modeling mass transport limitations in electrochemical systems.
- Migration Equations: Ion migration under an electric field also contributes to mass transport. Equations describing ion migration are important in certain electrochemical systems.
Conclusion: A Unified View of Charge in Electrochemistry
The equations described in this article provide a comprehensive framework for understanding the behavior of charge in electrochemical systems. From Faraday's Laws establishing the fundamental link between charge and mass, to the Nernst equation defining equilibrium potentials, and the Butler-Volmer equation detailing reaction kinetics, these equations are essential tools for researchers and engineers alike. Their applications span a wide range of fields, including energy storage, corrosion science, electroanalysis, and materials synthesis. A firm grasp of these equations is vital for anyone seeking a deeper understanding of the intricate world of electrochemistry. By mastering these principles, one can effectively model, predict, and control electrochemical processes. Further exploration into specialized areas like electrochemical impedance spectroscopy (EIS) would reveal even more sophisticated mathematical tools employed in this dynamic field.
Latest Posts
Latest Posts
-
Que Es La Descomposicion De Acidos
Mar 15, 2025
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Eqn For Charge In Electro Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
