Que Es La Descomposición De Acidos
Muz Play
Mar 15, 2025 · 5 min read
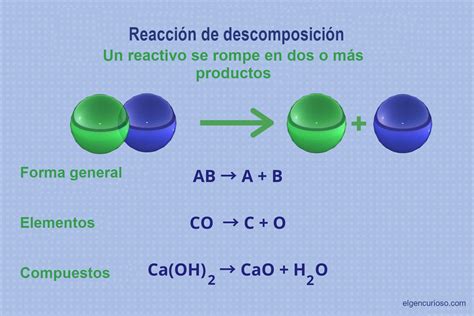
Table of Contents
What is Acid Decomposition? A Comprehensive Guide
Acid decomposition, often referred to as acid hydrolysis or acid degradation, is a chemical process where an acid reacts with a substance, breaking it down into smaller, simpler components. This process is widespread in various fields, from industrial chemistry to biological systems. Understanding acid decomposition requires examining the underlying chemical reactions, influencing factors, and its applications and implications.
The Chemistry of Acid Decomposition:
At its core, acid decomposition involves the protonation of a molecule or ion by an acid, followed by the cleavage of chemical bonds. The strength of the acid plays a crucial role; stronger acids facilitate more extensive decomposition. The nature of the substance being decomposed also dictates the reaction pathway and products.
Types of Acid Decomposition Reactions:
-
Ester Hydrolysis: This is a classic example where an ester reacts with water in the presence of an acid catalyst (like sulfuric acid). The ester bond breaks, yielding a carboxylic acid and an alcohol. This is commonly used in the production of soaps and other chemicals.
-
Amide Hydrolysis: Similar to ester hydrolysis, amides can be decomposed with acids. The amide bond breaks, producing a carboxylic acid and an amine. This is relevant in the breakdown of proteins and peptides.
-
Sugar Degradation: Sugars, particularly polysaccharides like cellulose and starch, undergo acid decomposition, producing smaller sugars and ultimately simple compounds like furfural. This process is crucial in the paper and pulp industry and is also relevant in food processing.
-
Polymer Degradation: Many polymers, both natural and synthetic, are susceptible to acid-catalyzed decomposition. The specific mechanism depends on the polymer's structure and the acid used. This can lead to depolymerization, generating smaller monomer units.
Factors Affecting Acid Decomposition:
Several factors can influence the rate and extent of acid decomposition:
-
Acid Strength: Stronger acids, such as sulfuric acid (H₂SO₄) and hydrochloric acid (HCl), generally lead to faster and more complete decomposition compared to weaker acids like acetic acid (CH₃COOH). The higher concentration of hydronium ions (H₃O⁺) in strong acids drives the reaction forward.
-
Concentration: A higher concentration of the acid accelerates the reaction rate. More acid molecules are available to interact with the substance undergoing decomposition.
-
Temperature: Increasing the temperature usually accelerates the rate of acid decomposition. The higher kinetic energy increases the frequency of collisions between acid molecules and the substance, leading to a higher reaction rate.
-
Reaction Time: Sufficient time is crucial for complete decomposition. The reaction rate might be fast initially, but it slows down as the reactants are consumed.
-
Catalyst Presence: Certain catalysts can enhance the rate of acid decomposition without being consumed in the process. These catalysts can lower the activation energy of the reaction.
-
Solvent: The choice of solvent can also affect the reaction rate and selectivity. Polar solvents generally facilitate acid-catalyzed reactions.
Applications of Acid Decomposition:
Acid decomposition finds widespread applications across diverse industries:
-
Food Processing: Acid hydrolysis is used in the production of corn syrup from starch, and in the preparation of certain food additives.
-
Pharmaceutical Industry: Acid decomposition is utilized in synthesizing many pharmaceutical compounds and in the degradation studies of drugs.
-
Paper and Pulp Industry: The acid hydrolysis of cellulose is key in breaking down wood pulp into smaller sugar molecules for various applications.
-
Textile Industry: Acid decomposition is sometimes employed in treating textiles to modify their properties.
-
Environmental Science: The acid decomposition of organic matter plays a vital role in nutrient cycling in the environment. It's also relevant in understanding environmental pollution from acid rain.
-
Chemical Synthesis: Acid decomposition is a cornerstone reaction in various organic synthesis processes. It's used to break down complex molecules into simpler building blocks for creating new chemicals.
Industrial Processes Utilizing Acid Decomposition:
Several industrial processes depend heavily on acid decomposition:
-
Production of Glucose from Starch: Starch, a complex carbohydrate, is subjected to acid hydrolysis to break it down into glucose, a simpler sugar widely used as a sweetener and in various other applications. This process often employs strong acids and elevated temperatures.
-
Hydrolysis of Fats and Oils: Fats and oils (triglycerides) are hydrolyzed using acids to yield fatty acids and glycerol. This process is crucial in the production of soaps and biodiesel.
-
Treatment of Waste Streams: Acid hydrolysis can be applied to break down certain organic compounds in wastewater, reducing its environmental impact. This process helps to manage and reduce pollution.
-
Production of Biofuels: Acid-catalyzed processes are being explored for the production of biofuels from various biomass sources. The acid hydrolysis of lignocellulosic biomass is a crucial step in releasing fermentable sugars for ethanol production.
Safety Precautions in Acid Decomposition:
Acid decomposition reactions often involve hazardous materials. Strict adherence to safety protocols is vital:
-
Personal Protective Equipment (PPE): Always use appropriate PPE, including safety goggles, gloves, lab coats, and respirators, when handling acids.
-
Ventilation: Adequate ventilation is essential to avoid inhaling hazardous fumes.
-
Careful Handling: Handle acids with care, avoiding spills and splashes.
-
Neutralization: If acid spills occur, neutralize the spill immediately with a suitable base, following established safety protocols.
-
Waste Disposal: Dispose of acid waste properly according to local regulations.
Environmental Implications of Acid Decomposition:
While acid decomposition has numerous beneficial applications, it also has environmental implications:
-
Acid Rain: Acid rain, caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere, leads to the acidification of soil and water bodies, harming ecosystems.
-
Pollution: Improper disposal of acid waste can contaminate soil and water, posing a threat to the environment and human health.
Further Research and Development:
Research continues on optimizing acid decomposition processes for increased efficiency and reduced environmental impact. Exploring greener alternatives and developing more sustainable methods are crucial. This includes research into:
-
Solid Acid Catalysts: These catalysts can be more easily recovered and reused, minimizing waste.
-
Enzyme-Catalyzed Hydrolysis: Enzymes can provide more selective and environmentally friendly alternatives to acid catalysts.
-
Process Intensification: This aims to enhance the efficiency and sustainability of acid decomposition processes.
In conclusion, acid decomposition is a fundamental chemical process with a broad range of applications across various industries. Understanding its underlying principles, influencing factors, and implications is critical for developing safe, efficient, and environmentally responsible practices. Continuous research and innovation are crucial to maximizing the benefits while mitigating the potential negative impacts of this important chemical process. This detailed exploration of acid decomposition provides a comprehensive overview, highlighting its significance in chemistry and beyond.
Latest Posts
Latest Posts
-
Columns Of The Periodic Table Are Called
Mar 15, 2025
-
Is Boiling Point Intensive Or Extensive
Mar 15, 2025
-
A Triple Bond Is Generally Composed Of
Mar 15, 2025
-
Actin Filaments Are Anchored To Structures Called
Mar 15, 2025
-
How To Identify A Weak Acid
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Que Es La Descomposición De Acidos . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
