Which One Neutral Charge Proton Or Neutron
Muz Play
Mar 15, 2025 · 5 min read
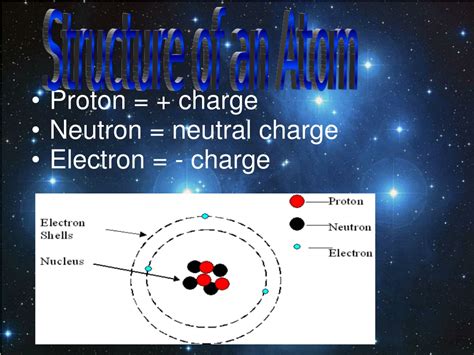
Table of Contents
Which One Has a Neutral Charge: Proton or Neutron?
The question of whether a proton or neutron carries a neutral charge is fundamental to understanding atomic structure and nuclear physics. The simple answer is: the neutron carries a neutral charge. However, a deeper dive reveals a more nuanced picture, exploring the internal structure of these subatomic particles and their roles in the nucleus.
Understanding Subatomic Particles
Atoms, the building blocks of matter, are composed of three primary subatomic particles: protons, neutrons, and electrons. Each plays a distinct role in determining an atom's properties:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element (e.g., one proton for hydrogen, two for helium).
- Neutrons: Neutrally charged particles also located in the atom's nucleus. Neutrons contribute to the atom's mass but not its charge. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons generally equals the number of protons in a neutral atom, balancing the positive charge of the protons.
The Charge of a Proton
A proton carries a positive charge, conventionally denoted as +1e, where 'e' represents the elementary charge (approximately 1.602 x 10^-19 Coulombs). This positive charge is an intrinsic property, meaning it's fundamental to the proton's nature and cannot be separated from it. The positive charge of protons is crucial for electrostatic interactions within the atom and with other charged particles.
The Quark Composition of a Proton
To understand the origin of a proton's positive charge, we need to delve into its internal structure. Protons are not fundamental particles; they are composed of three smaller particles called quarks. Specifically, a proton consists of two up quarks (each carrying a charge of +2/3e) and one down quark (carrying a charge of -1/3e). The sum of their charges (+2/3e + 2/3e - 1/3e) equals +1e, the overall positive charge of the proton.
The Charge of a Neutron
Unlike protons, neutrons possess no net electric charge. Their charge is precisely zero (0e). This neutrality is crucial for the stability of the atomic nucleus. The strong nuclear force, which overcomes the electrostatic repulsion between positively charged protons, is significantly influenced by the presence of neutrons.
The Quark Composition of a Neutron
Neutrons, similar to protons, are also composed of three quarks. However, their quark composition differs: a neutron comprises one up quark (+2/3e) and two down quarks (-1/3e each). Adding the charges (+2/3e - 1/3e - 1/3e) results in a net charge of 0e, confirming the neutron's neutral nature.
Why Neutrons are Crucial for Nuclear Stability
The presence of neutrons significantly impacts the stability of atomic nuclei, especially in heavier atoms. The strong nuclear force, responsible for binding protons and neutrons together in the nucleus, is short-range. As the number of protons increases, the electrostatic repulsion between them grows stronger, threatening to overcome the strong nuclear force and cause the nucleus to break apart.
Neutrons, by virtue of their neutral charge, help to mitigate this electrostatic repulsion. They increase the strong nuclear force's reach and strength without adding to the repulsive forces. This allows for the formation of stable nuclei with a larger number of protons. The optimal neutron-to-proton ratio for nuclear stability varies depending on the element.
Isotopes and Neutron Variations
The number of neutrons in an atom's nucleus can vary, even within the same element. These variations are known as isotopes. Isotopes of the same element have the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are radioactive, meaning they undergo decay to become more stable configurations.
For instance, carbon-12 (⁶C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are carbon isotopes, but ¹⁴C is radioactive and decays over time. The differing neutron counts influence the isotope's stability and properties.
Beyond the Basics: Magnetic Moments
While neutrons have no net electric charge, they do possess a magnetic moment. This means they behave like tiny magnets, interacting with external magnetic fields. This magnetic moment arises from the complex internal dynamics of the quarks within the neutron, even though the net charge is zero. The existence of a magnetic moment further highlights the intricate nature of these subatomic particles.
Neutron Decay and Beta Radiation
Although neutrons are stable within many atomic nuclei, free neutrons (those not bound within a nucleus) are unstable and decay through a process called beta decay. In this process, a neutron transforms into a proton, an electron (beta particle), and an antineutrino. This decay demonstrates the interconnectedness of subatomic particles and the dynamic nature of nuclear forces. The decay releases energy, making it a form of ionizing radiation.
Applications of Neutrons
The unique properties of neutrons, including their neutral charge, have led to their wide application in various fields:
- Nuclear reactors: Neutrons play a vital role in nuclear fission reactions, sustaining the chain reaction that produces energy.
- Neutron scattering: This technique uses neutron beams to study the structure and properties of materials at the atomic level, providing insights into crystal structures, molecular dynamics, and magnetic properties.
- Neutron activation analysis: This analytical technique uses neutrons to induce radioactivity in samples, allowing for the determination of elemental composition.
- Medical imaging: Neutron capture therapy is a developing technique used in cancer treatment.
Conclusion
In conclusion, the answer to the question "Which one has a neutral charge: proton or neutron?" is definitively the neutron. While protons carry a positive charge due to their quark composition, neutrons have a net charge of zero. However, this seemingly simple answer opens up a fascinating exploration of the internal structure of these subatomic particles, their roles in nuclear stability, their behavior in magnetic fields, and their crucial applications in various scientific and technological fields. The study of protons and neutrons remains a vibrant area of research, constantly pushing the boundaries of our understanding of the fundamental forces governing the universe.
Latest Posts
Latest Posts
-
The Electric Potential Energy Difference Between Two Points
Mar 15, 2025
-
Provide The Formula For Each Compound
Mar 15, 2025
-
Columns Of The Periodic Table Are Called
Mar 15, 2025
-
Is Boiling Point Intensive Or Extensive
Mar 15, 2025
-
A Triple Bond Is Generally Composed Of
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which One Neutral Charge Proton Or Neutron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
