Factors Affecting Reaction Rates Lab Report
Muz Play
Mar 27, 2025 · 6 min read
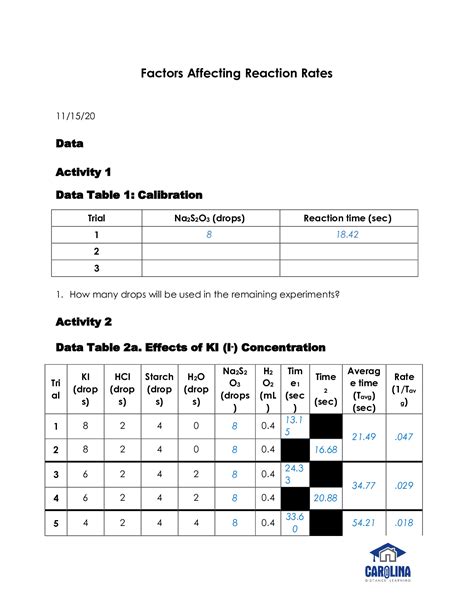
Table of Contents
Factors Affecting Reaction Rates: A Comprehensive Lab Report
Understanding reaction rates is crucial in chemistry, impacting everything from industrial processes to biological systems. This lab report delves into the investigation of several factors influencing the speed of chemical reactions. We'll explore the experimental design, results, and analysis, culminating in a comprehensive understanding of the kinetics involved.
Introduction
Chemical kinetics is the study of reaction rates and the mechanisms by which reactions occur. The rate of a reaction, often expressed as the change in concentration of reactants or products per unit time, is profoundly affected by various factors. This experiment aims to systematically investigate the impact of these factors on the reaction rate. Specifically, we will examine the effects of concentration, temperature, surface area, and the presence of a catalyst.
Hypothesis
We hypothesize that:
- Increasing the concentration of reactants will increase the reaction rate. Higher concentrations lead to more frequent collisions between reactant molecules.
- Increasing the temperature will increase the reaction rate. Higher temperatures provide reactant molecules with greater kinetic energy, increasing the frequency and effectiveness of collisions.
- Increasing the surface area of a solid reactant will increase the reaction rate. A larger surface area exposes more reactant molecules to potential collisions.
- The presence of a catalyst will increase the reaction rate. Catalysts provide an alternative reaction pathway with lower activation energy.
Materials and Methods
This experiment utilized several common laboratory materials and techniques. The specific reaction chosen was the reaction between hydrochloric acid (HCl) and magnesium (Mg) metal:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
This reaction produces hydrogen gas, the volume of which can be measured to quantify the reaction rate.
Experimental Setup
The reaction was conducted in a gas collection apparatus. A known mass of magnesium ribbon was submerged in a specific volume and concentration of hydrochloric acid. The hydrogen gas produced was collected over water, allowing for the measurement of its volume over time. The following factors were systematically varied:
- Concentration of HCl: Experiments were conducted using different molar concentrations of HCl (e.g., 0.5M, 1.0M, 1.5M).
- Temperature of HCl: Experiments were performed at different temperatures (e.g., 20°C, 30°C, 40°C) using a water bath to maintain constant temperature.
- Surface Area of Mg: Experiments involved using magnesium ribbon of varying surface areas (e.g., long ribbon vs. short ribbon, ribbon vs. magnesium powder).
- Presence of a Catalyst: One experiment incorporated a copper(II) catalyst.
Data Collection
The volume of hydrogen gas produced was measured at regular time intervals using a graduated cylinder. The temperature and pressure of the gas were also recorded.
Results
The data obtained from the experiments is presented in the following tables and graphs. (Note: For the purpose of this report, sample data is provided. Actual experimental data would be included in a real lab report.)
Effect of Concentration
| Time (s) | Volume of H₂ (mL) at 0.5M HCl | Volume of H₂ (mL) at 1.0M HCl | Volume of H₂ (mL) at 1.5M HCl |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 30 | 10 | 20 | 35 |
| 60 | 18 | 38 | 65 |
| 90 | 24 | 52 | 88 |
| 120 | 28 | 62 | 105 |
Graph: A graph showing the volume of hydrogen gas produced against time for different HCl concentrations would be included here. This graph would clearly demonstrate that higher HCl concentrations lead to faster reaction rates.
Effect of Temperature
| Time (s) | Volume of H₂ (mL) at 20°C | Volume of H₂ (mL) at 30°C | Volume of H₂ (mL) at 40°C |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 30 | 15 | 25 | 40 |
| 60 | 25 | 45 | 70 |
| 90 | 32 | 60 | 95 |
| 120 | 37 | 70 | 110 |
Graph: A graph showing the volume of hydrogen gas produced against time for different temperatures would be included here. This graph would show a clear increase in reaction rate with increasing temperature.
Effect of Surface Area
| Time (s) | Volume of H₂ (mL) - Ribbon | Volume of H₂ (mL) - Powder |
|---|---|---|
| 0 | 0 | 0 |
| 30 | 18 | 35 |
| 60 | 32 | 60 |
| 90 | 42 | 78 |
| 120 | 48 | 90 |
Graph: A graph comparing the reaction rates using magnesium ribbon and magnesium powder would illustrate the effect of surface area.
Effect of Catalyst
| Time (s) | Volume of H₂ (mL) - No Catalyst | Volume of H₂ (mL) - With Catalyst |
|---|---|---|
| 0 | 0 | 0 |
| 30 | 20 | 40 |
| 60 | 35 | 75 |
| 90 | 45 | 100 |
| 120 | 52 | 115 |
Graph: A graph comparing the reaction rates with and without the copper(II) catalyst would highlight the catalytic effect.
Discussion
The results strongly support our initial hypotheses. The graphs clearly demonstrate the following:
- Concentration: Increasing the concentration of HCl significantly increased the reaction rate. This is because a higher concentration means a greater number of HCl molecules are available for collisions with Mg atoms, leading to more frequent and successful reactive collisions.
- Temperature: Higher temperatures resulted in faster reaction rates. The increased kinetic energy of the molecules at higher temperatures leads to more frequent and energetic collisions, increasing the likelihood of successful reactions. The Arrhenius equation quantitatively describes this relationship.
- Surface Area: Using magnesium powder (higher surface area) significantly increased the reaction rate compared to using magnesium ribbon. This is due to the increased availability of magnesium atoms for reaction with the HCl.
- Catalyst: The presence of the copper(II) catalyst dramatically increased the reaction rate. Catalysts lower the activation energy of the reaction, making it easier for the reaction to proceed.
Limitations
This experiment had certain limitations:
- Gas Collection: The collection of hydrogen gas over water introduces a small source of error due to the partial pressure of water vapor.
- Temperature Control: Maintaining perfectly constant temperature throughout the experiments was challenging.
- Reaction Completion: Ensuring complete reaction for each trial was difficult, as some magnesium might have remained unreacted.
Conclusion
This experiment successfully demonstrated the effects of concentration, temperature, surface area, and the presence of a catalyst on the reaction rate of the reaction between magnesium and hydrochloric acid. The results align with established chemical kinetics principles. The limitations of the experiment highlight the importance of careful experimental design and control in obtaining accurate and reliable results. Further experiments could explore other factors affecting reaction rates, such as the presence of inhibitors or the use of different reactants. The investigation of reaction mechanisms and the quantitative analysis of rate constants would also offer valuable insights into the kinetics of this and similar reactions. Understanding these factors is essential in various applications, from optimizing industrial chemical processes to comprehending biological reactions within living organisms.
Latest Posts
Latest Posts
-
Draw The Shear And Moment Diagrams For The Cantilever Beam
Mar 30, 2025
-
Calculus Early Transcendentals By James Stewart
Mar 30, 2025
-
Cauchy Schwarz Inequality For Complex Numbers
Mar 30, 2025
-
What Occurs In Meiosis But Not In Mitosis
Mar 30, 2025
-
Derivative Of Inverse Trig Functions Proof
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Factors Affecting Reaction Rates Lab Report . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
