Fcc Unit Cell Number Of Atoms
Muz Play
Mar 25, 2025 · 6 min read
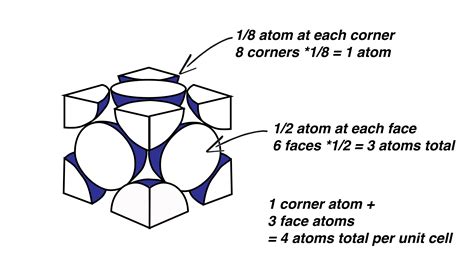
Table of Contents
FCC Unit Cell: A Deep Dive into the Number of Atoms
The face-centered cubic (FCC) unit cell is a fundamental building block in crystallography, representing the arrangement of atoms in many important metals and alloys. Understanding the number of atoms within an FCC unit cell is crucial for calculating density, determining material properties, and grasping the underlying principles of materials science. This article provides a comprehensive exploration of the FCC unit cell, meticulously detailing the calculation and implications of its atomic count.
Understanding the FCC Unit Cell Structure
Before delving into the atom count, let's establish a clear understanding of the FCC unit cell's geometry. Imagine a cube, which represents the unit cell. In an FCC arrangement, atoms are located at each of the cube's corners and at the center of each of its six faces. This arrangement leads to a highly efficient packing of atoms, maximizing the use of space.
Atom Locations within the FCC Unit Cell
-
Corner Atoms: Eight atoms are situated at each corner of the cube. Crucially, each corner atom is shared by eight adjacent unit cells. Therefore, only 1/8th of each corner atom belongs to a single unit cell.
-
Face-Centered Atoms: Six atoms reside at the center of each of the cube's six faces. Each face-centered atom is shared by two adjacent unit cells. Consequently, only ½ of each face-centered atom belongs to a single unit cell.
Calculating the Number of Atoms per FCC Unit Cell
Now, we can calculate the total number of atoms associated with a single FCC unit cell:
Corner atoms contribution: (8 corner atoms) * (1/8 atom/corner atom) = 1 atom
Face-centered atoms contribution: (6 face-centered atoms) * (1/2 atom/face-centered atom) = 3 atoms
Total atoms per unit cell: 1 atom + 3 atoms = 4 atoms
Therefore, a single FCC unit cell contains a total of four atoms. This is a key characteristic of the FCC structure and significantly impacts its properties.
Implications of the Four Atoms per Unit Cell
The fact that an FCC unit cell contains four atoms has far-reaching consequences across various material science disciplines. Let's explore some key implications:
Density Calculations
Knowing the number of atoms per unit cell is fundamental to calculating the material's density. The formula for density (ρ) is:
ρ = (n * M) / (V * N<sub>A</sub>)
Where:
- n = number of atoms per unit cell (in this case, 4 for FCC)
- M = molar mass of the element (g/mol)
- V = volume of the unit cell (cm³)
- N<sub>A</sub> = Avogadro's number (6.022 x 10²³ atoms/mol)
By knowing the unit cell's dimensions and the material's atomic weight, we can accurately determine its theoretical density. This is a crucial parameter in material selection and engineering.
Material Properties
The arrangement of atoms in the FCC structure directly influences the material's properties. The close-packed nature of the FCC lattice leads to:
- High Density: The efficient packing results in a relatively high density compared to other crystal structures.
- Ductility and Malleability: The ability of FCC metals to deform plastically under stress (ductility and malleability) stems from the ease with which atoms can slip past each other in this closely packed structure.
- High Electrical Conductivity: The close-packed structure also facilitates the efficient movement of electrons, contributing to good electrical conductivity.
- Thermal Conductivity: Similar to electrical conductivity, the FCC structure generally supports high thermal conductivity.
Examples of FCC Metals and Alloys
Numerous metals and alloys exhibit an FCC crystal structure, highlighting the prevalence and importance of this configuration. Some notable examples include:
- Aluminum (Al): A lightweight, strong, and highly conductive metal widely used in various applications.
- Copper (Cu): An excellent conductor of electricity and heat, crucial in electrical wiring and heat exchangers.
- Gold (Au): A precious metal known for its malleability, ductility, and inertness.
- Silver (Ag): Another highly conductive metal with applications in electronics and jewelry.
- Nickel (Ni): Used in various alloys due to its corrosion resistance and high strength.
- Platinum (Pt): A precious metal used in catalytic converters and jewelry.
- Austenitic Stainless Steels: A family of stainless steels with excellent corrosion resistance and formability. These steels owe their properties to their FCC structure.
These materials demonstrate the significant impact of the FCC crystal structure on the properties of numerous engineering materials.
Comparing FCC with Other Crystal Structures
To fully appreciate the significance of the four atoms per unit cell in FCC, let's briefly compare it to other common crystal structures:
Body-Centered Cubic (BCC)
In a BCC structure, atoms are located at the corners of a cube and one atom in the center of the cube. This results in a total of two atoms per unit cell. BCC metals generally have higher yield strength than FCC metals but lower ductility.
Simple Cubic (SC)
The simplest crystal structure, SC, has atoms only at the corners of the cube. This yields only one atom per unit cell. SC structures are relatively uncommon in metals due to their low packing efficiency.
Hexagonal Close-Packed (HCP)
HCP structures are also highly efficient in terms of atomic packing, like FCC. However, HCP's unit cell is more complex, resulting in six atoms. HCP metals often exhibit good strength and ductility, but usually less than FCC metals.
This comparison underscores the unique properties conferred by the four atoms per unit cell in the FCC structure, leading to its prevalence in many technologically important materials.
Advanced Concepts and Applications
The fundamental understanding of four atoms per FCC unit cell opens doors to more advanced concepts in materials science:
Crystallographic Directions and Planes
The FCC structure exhibits specific crystallographic directions and planes that influence material properties and deformation mechanisms. These are defined based on the arrangement of atoms within the unit cell. This is crucial for understanding things like slip systems and mechanical behavior.
Point Defects and Dislocations
Understanding the atomic arrangement within the FCC unit cell is vital in analyzing point defects (vacancies, interstitials) and dislocations (line defects) that affect material properties, such as strength and conductivity. The presence of defects disrupts the regular lattice arrangement.
Alloying and Phase Transformations
Alloying, the addition of other elements, can significantly alter the properties of FCC metals. Understanding the FCC structure is crucial to predicting the effects of alloying on the resultant material's microstructure and properties. Furthermore, phase transformations within FCC alloys, such as ordering or precipitation, are dependent on the atomic arrangement.
X-ray Diffraction and Characterization
X-ray diffraction (XRD) is a powerful technique used to determine the crystal structure of a material. The diffraction patterns obtained from an FCC material are unique and directly related to the number of atoms per unit cell and their arrangement.
Conclusion
The seemingly simple concept of four atoms per FCC unit cell is a cornerstone of materials science. Its impact resonates across various applications, shaping the properties of numerous crucial materials. From calculating density to understanding material behavior, this knowledge provides a foundation for advancements in materials engineering and research. A deep understanding of the FCC structure and its implications is essential for anyone working with metals and alloys.
Latest Posts
Latest Posts
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
-
What Does A Negative Enthalpy Mean
Mar 27, 2025
-
Divides The Body Into Anterior And Posterior Portions
Mar 27, 2025
-
Claim Of Fact Value And Policy
Mar 27, 2025
-
How To Calculate Shortage And Surplus
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Fcc Unit Cell Number Of Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
