Fes Compound Name With Roman Numerals
Muz Play
Mar 25, 2025 · 5 min read
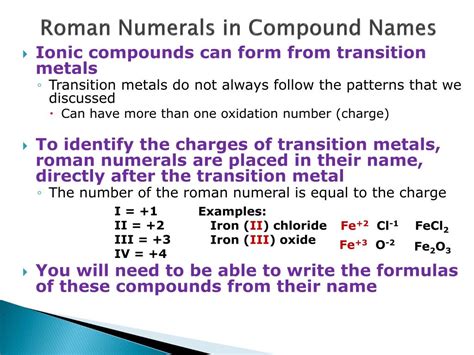
Table of Contents
Fes Compound Name with Roman Numerals: A Comprehensive Guide
The nomenclature of iron-sulfur (Fe-S) clusters, particularly those found in iron-sulfur proteins, often involves Roman numerals to indicate the number of iron atoms present. This naming convention, while seemingly straightforward, requires a nuanced understanding to accurately represent the complex structures and varying oxidation states of these essential biomolecules. This article provides a comprehensive guide to understanding Fe-S cluster naming conventions using Roman numerals, including their structural implications and biological significance.
Understanding Fe-S Clusters: Structure and Function
Iron-sulfur clusters are ubiquitous prosthetic groups found in a wide range of proteins across all domains of life. Their core structure consists of iron ions bridged by inorganic sulfide (S<sup>2-</sup>) ligands, often further coordinated by cysteine residues from the protein. The specific arrangement of iron and sulfur atoms, along with the protein environment, dictates the cluster's redox properties and biological function. These clusters play crucial roles in various metabolic processes, including:
- Electron Transfer: Many Fe-S proteins participate in electron transport chains, facilitating the movement of electrons during respiration and photosynthesis. The variable oxidation states of the iron atoms allow for reversible electron transfer.
- Catalysis: Some Fe-S clusters serve as catalytic centers in enzymes involved in crucial reactions like nitrogen fixation (nitrogenase) and the biosynthesis of various metabolites.
- Regulation: Fe-S clusters can act as regulatory switches, affecting protein activity in response to environmental changes or cellular signals.
The diversity in Fe-S cluster structures is reflected in the complexity of their nomenclature, with Roman numerals playing a key role in specifying the number of iron atoms involved.
Roman Numerals in Fe-S Cluster Nomenclature: A Detailed Explanation
The use of Roman numerals in Fe-S cluster names directly refers to the number of iron atoms (Fe) present in the cluster's core. For example:
- [Fe<sub>2</sub>S<sub>2</sub>]<sup>2+</sup> cluster: This is often referred to as a 2Fe-2S cluster, or simply a [2Fe-2S] cluster. The Roman numeral II represents the two iron atoms.
- [Fe<sub>3</sub>S<sub>4</sub>]<sup>1+</sup> cluster: This is termed a 3Fe-4S cluster or [3Fe-4S] cluster, indicating three iron atoms.
- [Fe<sub>4</sub>S<sub>4</sub>]<sup>2+</sup> cluster: This is known as a 4Fe-4S cluster or [4Fe-4S] cluster, clearly signifying four iron atoms.
It's important to note that the Roman numerals only refer to the number of iron atoms. The number of sulfide ions is often implied or stated explicitly, but it's not directly incorporated into the Roman numeral designation. The overall charge of the cluster is also a crucial characteristic and is typically indicated as a superscript.
Beyond Simple Roman Numeral Representation: Adding Nuance
While the basic Roman numeral system provides a fundamental framework for classification, the complexity of Fe-S clusters requires more detailed descriptions. Some factors influencing the nomenclature include:
- Isomerism: Some Fe-S clusters can exist in different isomeric forms, differing in the arrangement of iron and sulfur atoms. These isomers may require additional identifiers beyond the simple Roman numeral designation.
- Protein Context: The specific amino acid residues coordinating the cluster significantly impact its properties. The protein environment needs to be considered for a complete description. Often the name of the protein or enzyme containing the cluster is included.
- Oxidation State: The oxidation state of the iron atoms (e.g., Fe<sup>2+</sup>, Fe<sup>3+</sup>) is crucial for understanding the cluster's function. This information is usually included, often with a superscript indicating the net charge.
Common Fe-S Clusters and Their Nomenclature
Let's explore some of the most prevalent Fe-S cluster types and their nomenclature:
1. [2Fe-2S] Clusters (Fe<sub>II</sub>-Fe<sub>III</sub>)
These clusters are among the most common and are characterized by two iron atoms, each coordinated by two cysteine residues and bridged by two inorganic sulfide ions. The Roman numeral II highlights the two iron atoms. The oxidation states of the two iron atoms are typically a mixture of Fe<sup>II</sup> and Fe<sup>III</sup>, contributing to their redox activity. They are frequently found in electron transfer proteins.
2. [3Fe-4S] Clusters
These clusters contain three iron atoms and four inorganic sulfide ions. The arrangement of iron and sulfur atoms results in a unique structure with one iron atom exhibiting a slightly different coordination environment. Their redox properties are also important for electron transfer.
3. [4Fe-4S] Clusters
These are the largest and most complex commonly found Fe-S clusters, comprising four iron atoms and four sulfide ions. The four iron atoms are arranged in a cubane-like structure, coordinated by cysteine residues from the protein. The [4Fe-4S] cluster is highly versatile, exhibiting a wide range of redox potentials and frequently acting as electron carriers or catalytic centers.
Advanced Considerations and Future Directions
The field of Fe-S cluster research continues to evolve. New techniques, such as advanced spectroscopic methods and computational modeling, are revealing increasingly sophisticated details about cluster structures and their dynamic interactions within proteins. This necessitates ongoing refinement of the nomenclature system.
For instance, researchers are investigating:
- Novel Fe-S Cluster Structures: The discovery of unusual Fe-S clusters with unique structural arrangements requires expanding the current naming conventions.
- Dynamic Behavior: The study of Fe-S cluster dynamics, including their assembly, disassembly, and redox transitions, requires more descriptive and nuanced nomenclature to capture these changes.
- Biogenesis of Fe-S Clusters: Understanding the complex pathways and machinery involved in the biosynthesis of Fe-S clusters continues to be a significant area of research.
Conclusion
The use of Roman numerals in Fe-S cluster nomenclature provides a crucial foundation for understanding and communicating about these vital biomolecules. While the basic system is relatively straightforward, accurately describing these clusters requires consideration of structural nuances, redox states, and the protein environment. As research continues, the nomenclature may evolve to incorporate more detailed information, reflecting the growing understanding of this diverse and functionally important class of metalloclusters. This comprehensive guide provides a solid starting point for anyone seeking to understand the complex world of Fe-S clusters and their intricate nomenclature. Further research into the specific protein contexts and the intricate details of each individual cluster remains essential for complete comprehension. Ongoing advancements in analytical techniques and computational modeling promise to further illuminate this fascinating field, leading to a more precise and descriptive nomenclature system in the future.
Latest Posts
Latest Posts
-
What Is An Equation Of A Horizontal Line
Mar 26, 2025
-
What Is The Difference Between A Reactant And A Product
Mar 26, 2025
-
Neutralization Reaction Of Naoh And Hcl
Mar 26, 2025
-
Types Of Chemical Reactions Lab Answer Key
Mar 26, 2025
-
Double Angle And Half Angle Identities Worksheet
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Fes Compound Name With Roman Numerals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
