Final Electron Acceptor In Aerobic Respiration
Muz Play
Mar 18, 2025 · 6 min read
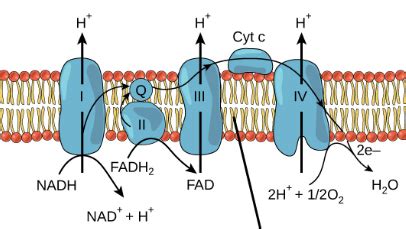
Table of Contents
The Final Electron Acceptor in Aerobic Respiration: Oxygen's Crucial Role in Energy Production
Aerobic respiration, the process by which cells break down glucose in the presence of oxygen to generate energy, is fundamental to life as we know it. Understanding this intricate process requires a detailed look at its various stages, including the crucial role of the final electron acceptor. This article delves deep into the function of oxygen as the ultimate electron acceptor in aerobic respiration, exploring its significance in ATP production and the consequences of its absence. We will also examine alternative electron acceptors used in anaerobic respiration and compare them to oxygen's efficiency.
The Electron Transport Chain: A Cascade of Electron Transfer
The electron transport chain (ETC), a series of protein complexes embedded within the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes), is the central stage where the magic of aerobic respiration unfolds. This chain facilitates a controlled cascade of electron transfer, gradually releasing energy that is harnessed to pump protons (H+) across the membrane, creating a proton gradient. This gradient is the driving force behind ATP synthesis, the process that generates the cell's primary energy currency, adenosine triphosphate.
The Role of Electron Carriers
Electrons, initially derived from the breakdown of glucose during glycolysis and the citric acid cycle, are carried to the ETC by electron carriers like NADH and FADH2. These molecules deliver their high-energy electrons to the various protein complexes within the chain. As electrons move down the chain, they pass through a series of redox reactions, losing energy at each step. This energy loss is not wasted; it's strategically used to pump protons across the inner mitochondrial membrane.
Establishing the Proton Motive Force
The pumping of protons creates an electrochemical gradient, also known as the proton motive force (PMF). This gradient consists of two components: a chemical gradient (difference in proton concentration) and an electrical gradient (difference in charge across the membrane). The PMF is essential because it stores the potential energy released during electron transport.
Oxygen: The Ultimate Electron Acceptor
At the end of the electron transport chain sits the final electron acceptor: oxygen (O2). Oxygen's high electronegativity makes it the ideal molecule to accept the low-energy electrons at the end of the chain. The acceptance of these electrons by oxygen is a critical step because it prevents the electron transport chain from becoming "blocked." Without a final electron acceptor, the chain would cease to function, halting ATP production.
The Formation of Water
When oxygen accepts the electrons, it combines with protons (H+) to form water (H2O). This is a crucial aspect of aerobic respiration, ensuring that the electrons are not simply accumulated within the chain, but are effectively removed and used to produce a harmless byproduct. The equation summarizing this reaction is:
4e- + 4H+ + O2 → 2H2O
This reaction is vital because it maintains the redox potential gradient needed for the continuous flow of electrons through the ETC. The continuous flow ensures the continual generation of the proton motive force and subsequent ATP synthesis.
ATP Synthase: Harnessing the Proton Gradient
The proton motive force, generated by the electron transport chain, drives ATP synthesis through a remarkable enzyme complex called ATP synthase. This molecular machine acts like a turbine, allowing protons to flow back across the inner mitochondrial membrane down their concentration gradient. This flow of protons rotates a part of the ATP synthase, causing a conformational change that catalyzes the synthesis of ATP from ADP and inorganic phosphate (Pi).
This process, known as chemiosmosis, is incredibly efficient, converting the potential energy stored in the proton gradient into the chemical energy stored in ATP. The vast majority of ATP produced during aerobic respiration is generated through this chemiosmotic coupling between the electron transport chain and ATP synthase.
Consequences of Oxygen Absence: Anaerobic Respiration
When oxygen is absent, aerobic respiration cannot occur because there is no final electron acceptor for the electron transport chain. In such conditions, cells resort to anaerobic respiration or fermentation. These processes are less efficient than aerobic respiration because they produce far less ATP.
Alternative Electron Acceptors in Anaerobic Respiration
In anaerobic respiration, different molecules act as the final electron acceptor. Examples include:
- Nitrate (NO3-): Used by some bacteria, reducing it to nitrite (NO2-) or even nitrogen gas (N2).
- Sulfate (SO42-): Used by sulfate-reducing bacteria, reducing it to hydrogen sulfide (H2S).
- Carbon dioxide (CO2): Used by methanogenic archaea, reducing it to methane (CH4).
- Fumarate: An organic molecule used by some bacteria in anaerobic conditions.
These alternative electron acceptors have lower electronegativity than oxygen, resulting in less energy being released during electron transfer. This directly translates to a significantly lower ATP yield compared to aerobic respiration.
Fermentation: A Cytoplasmic Process
Fermentation is a metabolic process that occurs in the cytoplasm without the involvement of an electron transport chain. It regenerates NAD+ from NADH, allowing glycolysis to continue even in the absence of oxygen. However, fermentation yields only a small amount of ATP (2 ATP molecules per glucose molecule), significantly less than aerobic respiration (approximately 30-36 ATP molecules). Examples of fermentation include lactic acid fermentation (producing lactic acid) and alcoholic fermentation (producing ethanol and carbon dioxide).
The Importance of Oxygen in Aerobic Respiration: A Summary
Oxygen's role as the final electron acceptor in aerobic respiration is absolutely critical for efficient energy production. Its high electronegativity allows for the complete oxidation of glucose, resulting in the maximum extraction of energy. Without oxygen, the electron transport chain becomes non-functional, leading to a drastic reduction in ATP production, hindering cellular processes and ultimately threatening the survival of aerobic organisms.
Comparative Analysis of Aerobic and Anaerobic Respiration
| Feature | Aerobic Respiration | Anaerobic Respiration |
|---|---|---|
| Electron Acceptor | Oxygen (O2) | Nitrate (NO3-), Sulfate (SO42-), CO2, etc. |
| ATP Yield | High (30-36 ATP per glucose molecule) | Low (2 ATP per glucose molecule, or less) |
| Location | Mitochondria (eukaryotes), plasma membrane (prokaryotes) | Cytoplasm |
| Electron Transport Chain | Present | May be absent (fermentation) |
| Efficiency | High | Low |
| Byproducts | Water (H2O) | Varies (e.g., lactic acid, ethanol, H2S) |
Beyond the Basics: Exploring Further
The intricacies of aerobic respiration extend beyond the fundamental concepts discussed here. Further research could explore:
- The regulation of the electron transport chain: How cellular processes control the rate of electron flow and ATP production based on the cell's energy demands.
- The role of reactive oxygen species (ROS): Oxygen's involvement in the production of harmful byproducts and the cellular mechanisms to mitigate their effects.
- The evolutionary implications of aerobic respiration: The significance of oxygen's emergence in Earth's atmosphere and its profound impact on the evolution of life.
- Clinical implications of mitochondrial dysfunction: The consequences of impaired electron transport chain function in various diseases.
Understanding the final electron acceptor in aerobic respiration is paramount to comprehending the fundamental principles of energy metabolism. The detailed knowledge of this process opens doors to further investigations in diverse fields, including medicine, biotechnology, and environmental science. The efficiency and impact of oxygen's role cannot be overstated; it is the cornerstone of the energy-generating processes that underpin life itself.
Latest Posts
Latest Posts
-
Which Base Is Not Found In Rna
Mar 18, 2025
-
So Long To Pinky Here Comes The Thumb
Mar 18, 2025
-
When Two Amino Acids Are Joined Together
Mar 18, 2025
-
What Is The Difference Between Chemical Reaction And Nuclear Reaction
Mar 18, 2025
-
How To Write Mass Balance Equations
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Final Electron Acceptor In Aerobic Respiration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
