Final Electron Acceptor In Electron Transport Chain
Muz Play
Apr 06, 2025 · 6 min read
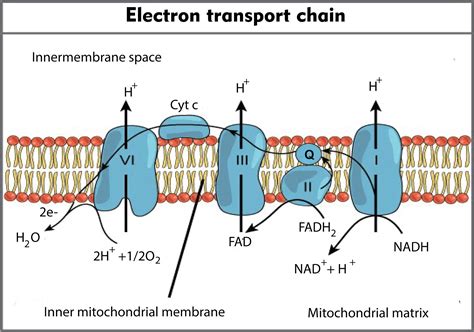
Table of Contents
The Final Electron Acceptor in the Electron Transport Chain: Oxygen and Beyond
The electron transport chain (ETC), a crucial component of cellular respiration, is responsible for generating the majority of ATP, the cell's energy currency. This intricate process involves a series of redox reactions where electrons are passed from one molecule to another, ultimately leading to the production of a proton gradient that drives ATP synthesis. Central to this process is the final electron acceptor, the molecule that receives the electrons at the end of the chain. While oxygen is the most common final electron acceptor in aerobic organisms, other molecules can fulfill this role in anaerobic environments. This article will delve deep into the role of the final electron acceptor, focusing primarily on oxygen, but also exploring alternative acceptors and their implications.
Oxygen: The Usual Suspect
In the vast majority of aerobic organisms, oxygen (O2) serves as the final electron acceptor in the electron transport chain. Its high electronegativity makes it an ideal candidate, readily accepting electrons and becoming reduced to water (H2O). This reduction reaction is critical because it prevents the electron transport chain from becoming "backed up," ensuring the continued flow of electrons and the generation of ATP.
The Reduction of Oxygen: A Detailed Look
The process of oxygen reduction doesn't occur in a single step. Instead, it involves a series of intermediate steps, catalyzed by cytochrome c oxidase, the final enzyme complex in the ETC. This complex contains several metal ions, including copper and iron, which facilitate the transfer of electrons to oxygen.
The overall reaction can be summarized as:
4e- + 4H+ + O2 → 2H2O
This reaction is essential for several reasons:
- Proton Gradient Maintenance: The consumption of protons (H+) during the reduction of oxygen contributes to the proton gradient across the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). This gradient is vital for ATP synthesis via chemiosmosis.
- Preventing Oxidative Stress: Without a final electron acceptor, electrons would accumulate in the ETC, potentially leading to the formation of reactive oxygen species (ROS), which are damaging to cellular components. Oxygen's efficient acceptance of electrons mitigates this risk.
- Efficient Energy Production: The high electronegativity of oxygen allows for the extraction of a significant amount of energy from the electron transport chain, maximizing ATP production.
The Importance of Oxygen in Aerobic Respiration
Oxygen's role as the final electron acceptor is inextricably linked to the high efficiency of aerobic respiration. Anaerobic processes, which use alternative electron acceptors, typically produce significantly less ATP per glucose molecule. This is because the reduction potential of oxygen is higher than that of other electron acceptors, resulting in a greater energy yield. The reliance of most complex life forms on oxygen highlights its critical role in supporting energy-demanding processes.
Alternative Final Electron Acceptors: Anaerobic Respiration
While oxygen is the preferred final electron acceptor, many organisms have evolved to utilize alternative acceptors in anaerobic environments lacking oxygen. This process is known as anaerobic respiration, and it involves a variety of different electron acceptors, each with its unique characteristics and energy yield.
Nitrate (NO3-) Reduction: A Common Alternative
Nitrate (NO3-) is a common alternative final electron acceptor used by many bacteria and archaea. The reduction of nitrate involves a series of steps, ultimately leading to the formation of nitrite (NO2-), nitric oxide (NO), nitrous oxide (N2O), or dinitrogen gas (N2). The specific end product depends on the enzymes present in the organism.
The reduction of nitrate is less energetically favorable than the reduction of oxygen, resulting in a lower ATP yield. However, it provides a means for survival in oxygen-depleted environments. The process is also important in the nitrogen cycle, playing a significant role in nitrogen transformations in the environment.
Sulfate (SO42-) Reduction: Energy from Sulfur
Sulfate (SO42-) reduction is another important anaerobic respiration pathway used by various microorganisms. These organisms, often referred to as sulfate-reducing bacteria (SRB), use sulfate as the terminal electron acceptor, reducing it to hydrogen sulfide (H2S). This process is crucial in sulfur cycling and contributes significantly to the formation of sedimentary deposits containing sulfides.
Similar to nitrate reduction, sulfate reduction is less efficient than oxygen reduction, yielding less ATP. The resulting hydrogen sulfide can be toxic in high concentrations and contributes to the characteristic smell of anaerobic environments like swamps and marshes.
Other Alternative Acceptors: Expanding the Possibilities
Besides nitrate and sulfate, several other molecules can act as final electron acceptors in anaerobic respiration, including:
- Fumarate: This organic molecule is reduced to succinate.
- Carbon Dioxide (CO2): Used by methanogenic archaea to produce methane (CH4). This is an important process in anaerobic ecosystems like wetlands.
- Iron (Fe3+): Reduced to ferrous iron (Fe2+). This process is significant in the geological cycling of iron.
- Manganese (Mn4+): Reduced to manganese (Mn2+).
The choice of alternative electron acceptor often depends on the availability of the acceptor in the environment and the metabolic capabilities of the organism. Each alternative pathway offers a different energy yield and contributes uniquely to the biogeochemical cycles of various elements.
The Impact of Final Electron Acceptor on ATP Production
The final electron acceptor profoundly impacts the efficiency of the electron transport chain and the overall ATP yield of cellular respiration. The reduction potential of the acceptor directly correlates with the amount of energy released during electron transport. Oxygen, with its high reduction potential, facilitates a substantial proton gradient, leading to high ATP production. In contrast, alternative acceptors have lower reduction potentials, resulting in a smaller proton gradient and consequently lower ATP yield.
This difference in ATP production has significant ecological implications. Aerobic organisms, relying on oxygen, thrive in environments with abundant oxygen and can support higher metabolic rates. Anaerobic organisms, utilizing alternative acceptors, are adapted to oxygen-poor environments and possess lower metabolic rates. The diversity of electron acceptors highlights the remarkable adaptability of life to various environmental conditions.
The Significance of Studying Final Electron Acceptors
Understanding the role of final electron acceptors in cellular respiration is essential for several reasons:
- Ecological Studies: Identifying the final electron acceptors used by microorganisms in various environments helps to understand the biogeochemical cycles of different elements and the functioning of various ecosystems.
- Bioremediation: Knowledge of alternative electron acceptors can be exploited in bioremediation strategies, where microorganisms are used to remove pollutants from contaminated environments.
- Medical Applications: Understanding the role of oxygen and its potential substitutes in cellular respiration helps us understand the pathogenesis of various diseases and develop new therapeutic approaches.
- Biotechnology: Harnessing the metabolic capabilities of microorganisms that utilize alternative electron acceptors can lead to the development of new biotechnologies for energy production and waste treatment.
Conclusion: A Versatile and Vital Process
The electron transport chain, with its diverse final electron acceptors, is a remarkable feat of biological engineering. The adaptability of organisms to utilize different acceptors showcases the resilience of life and highlights the importance of understanding the intricate interplay between metabolism, environment, and evolution. Oxygen remains the most efficient final electron acceptor, driving the high-energy requirements of aerobic life. However, the existence of alternative acceptors allows life to persist in environments where oxygen is scarce, showcasing the profound diversity and adaptability of life on Earth. Further research into the mechanisms and implications of different final electron acceptors promises to uncover even more about the fundamental processes of life and their impact on our planet.
Latest Posts
Latest Posts
-
What Phase Do Cells Spend Most Of Their Time In
Apr 07, 2025
-
Is Ionic Between Metal And Nonmetal
Apr 07, 2025
-
Is Tap Water A Heterogeneous Mixture
Apr 07, 2025
-
How To Describe Distribution In Statistics
Apr 07, 2025
-
Identify The Functional Groups In The Following Molecules
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Final Electron Acceptor In Electron Transport Chain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
