Finding The Molarity Of A Titration
Muz Play
Mar 29, 2025 · 5 min read
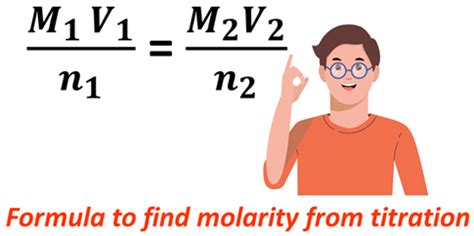
Table of Contents
- Finding The Molarity Of A Titration
- Table of Contents
- Finding the Molarity of a Titration: A Comprehensive Guide
- Understanding the Fundamentals: Molarity and Titration
- Molarity (M)
- Titration Process
- Types of Titrations
- Calculating Molarity from Titration Data: A Step-by-Step Guide
- Handling Different Stoichiometries
- Common Errors and Troubleshooting
- Advanced Techniques and Considerations
- Conclusion: Mastering Titration for Accurate Molarity Determination
- Latest Posts
- Latest Posts
- Related Post
Finding the Molarity of a Titration: A Comprehensive Guide
Titration is a fundamental technique in chemistry used to determine the concentration of an unknown solution, often referred to as the analyte, by reacting it with a solution of known concentration, called the titrant. Understanding how to calculate the molarity of a solution from titration data is crucial for accurate analytical chemistry. This comprehensive guide will walk you through the process, covering the theoretical background, practical steps, and common pitfalls to avoid.
Understanding the Fundamentals: Molarity and Titration
Before diving into calculations, let's refresh our understanding of key concepts.
Molarity (M)
Molarity is a unit of concentration, defined as the number of moles of solute per liter of solution. The formula is:
Molarity (M) = moles of solute / liters of solution
Therefore, to find molarity, you need to know both the number of moles of the solute and the volume of the solution.
Titration Process
Titration involves gradually adding the titrant from a burette to the analyte in a flask, typically using an indicator to signal the endpoint. The endpoint is the point at which the indicator changes color, indicating that the reaction between the titrant and analyte is complete (or close to complete). The volume of titrant used to reach the endpoint is crucial for molarity calculations.
Types of Titrations
Several types of titrations exist, each tailored to specific chemical reactions:
-
Acid-base titrations: These involve reacting an acid with a base, commonly using indicators like phenolphthalein or methyl orange. The reaction's stoichiometry dictates the mole ratio between acid and base.
-
Redox titrations: These involve the transfer of electrons between an oxidizing agent and a reducing agent. The endpoint is often determined using a redox indicator or potentiometric titration.
-
Complexometric titrations: These involve the formation of a complex between a metal ion and a chelating agent. EDTA is a common chelating agent used in these titrations.
-
Precipitation titrations: These involve the formation of a precipitate. The endpoint is often determined visually or using an indicator that changes color when the precipitation is complete.
Calculating Molarity from Titration Data: A Step-by-Step Guide
The core of determining molarity from a titration lies in understanding stoichiometry and using the balanced chemical equation. Here's a detailed step-by-step guide, focusing on acid-base titrations for clarity:
Step 1: Write a Balanced Chemical Equation
This is the foundation of all calculations. Let's consider a common example: the titration of a sodium hydroxide (NaOH) solution with a standardized hydrochloric acid (HCl) solution. The balanced equation is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation shows a 1:1 mole ratio between HCl and NaOH. This ratio is crucial for the next step.
Step 2: Determine the Moles of Titrant Used
Using the known molarity (M) and volume (V) of the titrant (HCl in our example), calculate the moles of titrant used:
Moles of titrant = Molarity (M) × Volume (L)
Remember to convert the volume from milliliters (mL) to liters (L) by dividing by 1000.
For example, if 25.00 mL of 0.100 M HCl was used, the moles of HCl would be:
Moles of HCl = 0.100 mol/L × (25.00 mL / 1000 mL/L) = 0.00250 moles
Step 3: Determine the Moles of Analyte
Using the balanced chemical equation's stoichiometry, determine the moles of analyte (NaOH in our example) that reacted with the titrant. In our 1:1 mole ratio example:
Moles of analyte = Moles of titrant × (Mole ratio of analyte to titrant from balanced equation)
Since the mole ratio is 1:1, the moles of NaOH are equal to the moles of HCl: 0.00250 moles.
Step 4: Calculate the Molarity of the Analyte
Finally, calculate the molarity of the analyte using the moles of analyte and the volume of the analyte solution used:
Molarity of analyte = Moles of analyte / Volume of analyte (L)
For example, if 20.00 mL of NaOH solution was titrated, the molarity would be:
Molarity of NaOH = 0.00250 moles / (20.00 mL / 1000 mL/L) = 0.125 M
Therefore, the concentration of the NaOH solution is 0.125 M.
Handling Different Stoichiometries
Not all titrations have a 1:1 mole ratio. Consider the titration of sulfuric acid (H₂SO₄) with sodium hydroxide (NaOH):
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
Notice the 1:2 mole ratio between H₂SO₄ and NaOH. In this case, Step 3 would be modified:
Moles of H₂SO₄ = Moles of NaOH × (1 mole H₂SO₄ / 2 moles NaOH)
Common Errors and Troubleshooting
Several factors can affect the accuracy of titration results:
-
Incorrect Endpoint Detection: Improper use of indicators or failure to observe the color change accurately can lead to significant errors. Practice and careful observation are key.
-
Improper Calibration of Equipment: Using uncalibrated glassware (burettes, pipettes) will introduce volumetric errors directly impacting molarity calculations. Always ensure proper calibration.
-
Parallax Error: Incorrect reading of the burette meniscus due to parallax can lead to inaccurate volume measurements. Always read the meniscus at eye level.
-
Contamination: Contamination of solutions or glassware can affect the reaction stoichiometry and lead to incorrect results. Ensure cleanliness and proper handling of reagents.
-
Incomplete Reaction: Failure to allow sufficient time for the reaction to complete before endpoint detection can result in an inaccurate molarity.
Advanced Techniques and Considerations
-
Back Titration: This technique is useful when the analyte reacts slowly or the endpoint is difficult to observe directly. An excess of titrant is added to the analyte, and then the excess titrant is titrated with another standard solution.
-
Potentiometric Titration: This technique uses an electrode to monitor the potential difference during the titration, providing a more precise determination of the endpoint. This method is particularly valuable for titrations with indistinct color changes.
-
Using Different Indicators: The choice of indicator depends on the pH range of the equivalence point. Different indicators have different pH transition ranges, so selecting the appropriate one is crucial.
Conclusion: Mastering Titration for Accurate Molarity Determination
Mastering titration techniques and understanding the associated calculations are essential skills for analytical chemists. This guide provided a comprehensive overview of the process, focusing on acid-base titrations but highlighting the broader applications and potential challenges. By carefully following the steps, understanding stoichiometry, and paying attention to potential errors, you can confidently determine the molarity of unknown solutions through titration and improve your analytical chemistry capabilities. Remember to always practice diligently and carefully review your calculations. Accuracy in titration is directly tied to accuracy in your results and conclusions.
Latest Posts
Latest Posts
-
Activation Energy For The Forward Reaction
Apr 02, 2025
-
Base Excision Repair Vs Mismatch Repair
Apr 02, 2025
-
Is Argon Metal Nonmetal Or Metalloid
Apr 02, 2025
-
What Is A Limiting Amino Acid In A Protein
Apr 02, 2025
-
Under What Conditions Are Gases Most Likely To Behave Ideally
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Finding The Molarity Of A Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
