First Order Reaction Vs Second Order Reaction
Muz Play
Mar 22, 2025 · 6 min read
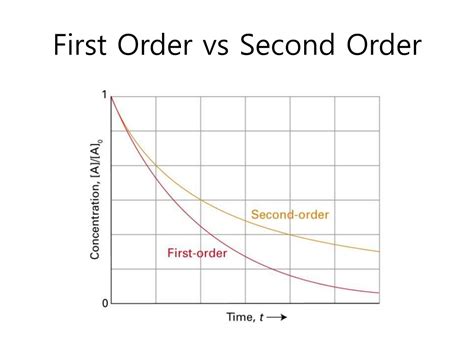
Table of Contents
First Order Reaction vs. Second Order Reaction: A Comprehensive Guide
Understanding reaction kinetics is fundamental in chemistry and related fields. A key aspect of this understanding lies in differentiating between various reaction orders, particularly first-order and second-order reactions. While both describe the rate at which reactants are consumed to form products, their underlying mechanisms and mathematical descriptions differ significantly. This article delves into the core concepts of first-order and second-order reactions, comparing and contrasting their characteristics, and providing illustrative examples.
Defining Reaction Order
Before diving into the specifics of first and second-order reactions, let's establish a clear understanding of reaction order itself. The reaction order defines the relationship between the rate of a chemical reaction and the concentration of the reactants. It's crucial to understand that reaction order is not necessarily related to the stoichiometric coefficients in the balanced chemical equation. It's determined experimentally.
The general rate law for a reaction is expressed as:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>
Where:
- Rate: The speed at which the reaction proceeds.
- k: The rate constant (specific to the reaction and temperature).
- [A] and [B]: The concentrations of reactants A and B.
- m and n: The orders of the reaction with respect to reactants A and B, respectively. These are experimentally determined exponents.
The overall reaction order is the sum of the individual orders (m + n).
First-Order Reactions: A Detailed Look
A first-order reaction is one where the rate of the reaction is directly proportional to the concentration of only one reactant. The rate law for a first-order reaction with reactant A is:
Rate = k[A]
Here, the reaction order with respect to A is 1, and the overall reaction order is also 1.
Characteristics of First-Order Reactions:
- Linear relationship: A plot of ln[A] versus time yields a straight line with a slope of -k. This is crucial for determining the rate constant experimentally.
- Half-life: The half-life (t<sub>1/2</sub>), the time it takes for the concentration of the reactant to decrease by half, is constant and independent of the initial concentration. The equation for the half-life of a first-order reaction is: t<sub>1/2</sub> = 0.693/k
- Exponential decay: The concentration of the reactant decreases exponentially with time.
- Examples: Radioactive decay, the decomposition of many organic molecules, and some enzyme-catalyzed reactions follow first-order kinetics.
Integrated Rate Law for First-Order Reactions:
The integrated rate law allows us to determine the concentration of the reactant at any given time:
ln[A]<sub>t</sub> = ln[A]<sub>0</sub> - kt
Where:
- [A]<sub>t</sub> is the concentration of A at time t.
- [A]<sub>0</sub> is the initial concentration of A.
- k is the rate constant.
Second-Order Reactions: A Comprehensive Analysis
A second-order reaction is one where the rate of the reaction is proportional to the concentration of two reactants, each raised to the power of one, or to the square of the concentration of a single reactant. There are two common scenarios:
1. Second-Order Reaction with Two Different Reactants:
The rate law is:
Rate = k[A][B]
Here, the reaction is first-order with respect to both A and B, and the overall reaction order is 2.
2. Second-Order Reaction with One Reactant:
The rate law is:
Rate = k[A]<sup>2</sup>
Here, the reaction is second-order with respect to A, and the overall reaction order is 2.
Characteristics of Second-Order Reactions:
- Non-linear relationship: A plot of 1/[A] versus time yields a straight line with a slope of k for the case of a second-order reaction with one reactant. For the case of two reactants, the analysis is more complex.
- Half-life dependence: The half-life of a second-order reaction is dependent on the initial concentration of the reactant(s). The half-life equation for a second-order reaction with one reactant is: t<sub>1/2</sub> = 1/(k[A]<sub>0</sub>)
- Non-exponential decay: The concentration of the reactant decreases more slowly than in a first-order reaction.
Integrated Rate Laws for Second-Order Reactions:
The integrated rate law for a second-order reaction with one reactant is:
1/[A]<sub>t</sub> = 1/[A]<sub>0</sub> + kt
For a second-order reaction with two different reactants (A and B), if the initial concentrations are significantly different ([B]<sub>0</sub> >> [A]<sub>0</sub>), then the reaction can be treated as pseudo-first-order.
Comparing First-Order and Second-Order Reactions: A Table Summary
| Feature | First-Order Reaction | Second-Order Reaction (one reactant) |
|---|---|---|
| Rate Law | Rate = k[A] | Rate = k[A]² |
| Units of k | s<sup>-1</sup> | M<sup>-1</sup>s<sup>-1</sup> |
| Half-life | t<sub>1/2</sub> = 0.693/k | t<sub>1/2</sub> = 1/(k[A]<sub>0</sub>) |
| Concentration vs. Time Plot | ln[A] vs. t is linear | 1/[A] vs. t is linear |
| Half-life Dependence | Independent of initial concentration | Dependent on initial concentration |
| Integrated Rate Law | ln[A]<sub>t</sub> = ln[A]<sub>0</sub> - kt | 1/[A]<sub>t</sub> = 1/[A]<sub>0</sub> + kt |
Determining Reaction Order Experimentally
The reaction order isn't determined by looking at the balanced chemical equation. Instead, it's determined experimentally through methods like:
- Method of initial rates: This involves measuring the initial rate of reaction at different initial concentrations of reactants. By comparing the changes in rate with changes in concentration, the order with respect to each reactant can be determined.
- Graphical method: Plotting different functions of concentration against time (e.g., ln[A] vs. t, 1/[A] vs. t) and identifying which plot yields a straight line indicates the reaction order.
Real-world Applications
Understanding the differences between first-order and second-order reactions is crucial in various fields:
- Pharmacokinetics: Determining the rate at which drugs are metabolized and eliminated from the body.
- Chemical engineering: Designing and optimizing chemical reactors.
- Environmental science: Modeling the degradation of pollutants in the environment.
- Nuclear chemistry: Predicting the decay rates of radioactive isotopes.
Advanced Concepts and Considerations
This article provides a foundational understanding of first and second order reactions. However, more complex scenarios exist:
- Fractional order reactions: Some reactions exhibit non-integer orders.
- Zero-order reactions: The rate is independent of reactant concentration.
- Complex reactions: Reactions involving multiple steps and intermediates.
- Temperature dependence: The rate constant (k) is highly temperature-dependent, often following the Arrhenius equation.
Conclusion
The distinction between first-order and second-order reactions is a cornerstone of chemical kinetics. While both describe the rate of a reaction, their mathematical descriptions, half-life characteristics, and experimental determination methods differ significantly. Mastering these concepts is essential for understanding reaction mechanisms and predicting the behavior of chemical systems in various applications. The ability to distinguish and analyze these reaction orders is vital for scientists and engineers in numerous fields. Further exploration of advanced concepts within reaction kinetics will build upon this foundational knowledge.
Latest Posts
Latest Posts
-
Organic Chemistry Substitution And Elimination Reactions
Mar 23, 2025
-
Arteries Of Lower Limb Flow Chart
Mar 23, 2025
-
What Is The Relation Between Inertia And Mass
Mar 23, 2025
-
1 2 Addition 1 4 Addition
Mar 23, 2025
-
What Is A Row Called In The Periodic Table
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about First Order Reaction Vs Second Order Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
