What Is A Row Called In The Periodic Table
Muz Play
Mar 23, 2025 · 6 min read
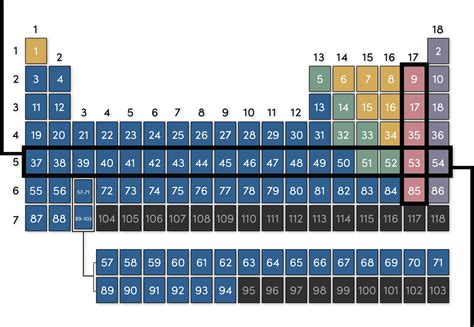
Table of Contents
What is a Row Called in the Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While most are familiar with the columns, called groups or families, understanding what a row in the periodic table is called is crucial for grasping the fundamental principles of chemical behavior and trends. This comprehensive guide delves deep into the concept of periods in the periodic table, exploring their significance and how they relate to an element's properties.
Understanding Periods in the Periodic Table: A Horizontal Journey
A row in the periodic table is called a period. Each period represents a principal energy level or shell in an atom. As you move across a period from left to right, the atomic number increases, meaning the number of protons and electrons in the atom also increases. This increase in electrons systematically fills the electron shells, leading to predictable changes in the chemical and physical properties of the elements within that period.
The Significance of Electron Shells and Periods
The arrangement of electrons in shells determines an element's reactivity and bonding characteristics. Each period corresponds to a specific number of electron shells. For example:
- Period 1: Contains only two elements, hydrogen (H) and helium (He), both with electrons filling the first electron shell (n=1).
- Period 2: Includes eight elements, from lithium (Li) to neon (Ne), with electrons progressively filling the second electron shell (n=2). This includes the 2s and 2p subshells.
- Period 3: Also contains eight elements, from sodium (Na) to argon (Ar), filling the third electron shell (n=3), encompassing 3s and 3p subshells.
This pattern continues, with each subsequent period adding another electron shell and thus increasing the complexity of the atom's electronic structure. However, the number of elements in each period isn't always eight due to the filling of d and f subshells in higher periods.
Periodicity and Trends Across Periods
The periodic table's structure reflects the periodic law, which states that the properties of elements are a periodic function of their atomic number. As you move across a period, several key properties exhibit predictable trends:
1. Atomic Radius: A Shrinking Trend
Atomic radius, the distance from the atom's nucleus to its outermost electron, generally decreases across a period. This is because the number of protons in the nucleus increases, resulting in a stronger positive charge attracting the electrons closer to the nucleus. Although the number of electrons increases, the additional electrons are added to the same principal energy level, experiencing increased nuclear pull, outweighing the shielding effect.
2. Ionization Energy: The Energy Required for Removal
Ionization energy is the energy required to remove an electron from a neutral atom. Ionization energy generally increases across a period. This is directly linked to the increased nuclear charge and decreased atomic radius. The stronger attraction between the nucleus and electrons makes it more difficult to remove an electron.
3. Electronegativity: The Pull of Electrons
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period because of the increased nuclear charge, making atoms more likely to attract electrons from other atoms.
4. Electron Affinity: Adding an Electron
Electron affinity is the energy change associated with adding an electron to a neutral atom. It generally increases across a period, although with some exceptions. This increase reflects the increased attractive force of the nucleus for the additional electron.
5. Metallic Character: A Gradual Shift
Metallic character, representing the ability of an element to lose electrons, generally decreases across a period. Elements at the beginning of periods (alkali and alkaline earth metals) are highly metallic, readily losing electrons to form positive ions. As you move across to the right, nonmetallic character increases, with elements tending to gain electrons rather than lose them.
Exceptions and Irregularities: A Closer Look
While the general trends described above are consistent, some exceptions exist. These deviations are often attributed to electron configurations and shielding effects. For example, the electron configuration of elements can influence their properties, leading to irregularities in trends. The subtle nuances of electron-electron repulsion and the complexities of electron shielding can also lead to deviations from the expected trends.
The Role of Subshells: Beyond the Simple Trends
The explanation of periodic trends across periods becomes more intricate when considering subshells. The filling of different subshells (s, p, d, and f) within a principal energy level influences the electronic structure and the properties of the elements. The gradual filling of these subshells with electrons explains the variations in the properties of elements within a period. For example, the transition metals (d-block elements) exhibit unique characteristics because of the filling of the d-subshell. Similarly, the lanthanides and actinides (f-block elements) show properties influenced by the filling of the f-subshell.
The Significance of Periodicity: Applications and Implications
Understanding the concepts of periods and periodicity has broad implications in various fields, including:
- Predicting Chemical Properties: Knowing an element's period helps predict its chemical behavior, reactivity, and bonding characteristics.
- Material Science: The periodicity of properties is crucial in designing and developing new materials with specific characteristics.
- Industrial Chemistry: The properties of elements within a period are essential in selecting appropriate elements for various industrial applications, such as catalysis and metallurgy.
- Biological Systems: The periodic table and its principles are essential to understanding the role of elements in biological systems and processes.
Beyond the Basics: Advanced Concepts in Periodicity
The concept of periods extends beyond the simple trends discussed above. More advanced considerations include:
- Effective Nuclear Charge: This considers the net positive charge experienced by an electron, accounting for the shielding effect of other electrons.
- Electron Shielding: This describes how inner electrons reduce the attraction between the nucleus and outer electrons.
- Quantum Mechanical Models: These provide a more accurate description of electron behavior and help explain the nuances of periodic trends.
Understanding these advanced concepts provides a deeper insight into the complexities of atomic structure and chemical behavior.
Conclusion: Periods – A Fundamental Concept in Chemistry
In summary, a row in the periodic table is called a period. Periods represent principal energy levels, and the progressive filling of electron shells within each period leads to the observed periodicity of element properties. Grasping this fundamental concept is vital for comprehending the organization and behavior of elements, paving the way for a deeper understanding of chemistry and its many applications. By appreciating the trends and irregularities within periods, we can effectively predict chemical properties and unlock the potential of elements for various applications across numerous scientific and technological fields. The periodic table, with its periods and groups, remains a powerful tool for exploring the fascinating world of chemistry and its endless possibilities. Further investigation into the intricacies of each period and the unique properties of elements within will continue to reveal new insights and applications.
Latest Posts
Latest Posts
-
Table Of Standard Heats Of Formation
Mar 24, 2025
-
What Are The Units Of Heat
Mar 24, 2025
-
Conservation Of Power In A Circuit
Mar 24, 2025
-
What Is The Relationship Between Absorbance And Concentration
Mar 24, 2025
-
How To Multiply Vector Wise Equations
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is A Row Called In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
