What Is The Relationship Between Absorbance And Concentration
Muz Play
Mar 24, 2025 · 7 min read
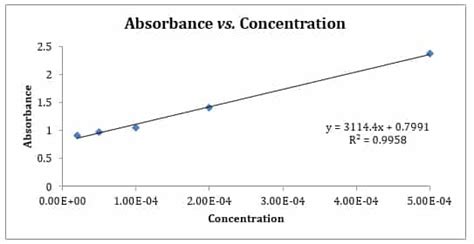
Table of Contents
The Relationship Between Absorbance and Concentration: A Deep Dive into Beer-Lambert Law
Understanding the relationship between absorbance and concentration is fundamental in various scientific fields, particularly in analytical chemistry and spectroscopy. This relationship is precisely defined by the Beer-Lambert Law, a cornerstone principle that governs the attenuation of light as it passes through a solution. This article will explore this law in detail, examining its underlying principles, limitations, and practical applications. We'll delve into the factors influencing absorbance, explore deviations from the law, and discuss how this relationship is utilized in various analytical techniques.
Understanding the Beer-Lambert Law
The Beer-Lambert Law, also known as the Beer-Lambert-Bouguer Law, states that the absorbance of a solution is directly proportional to the concentration of the analyte and the path length of the light through the solution. Mathematically, it's represented as:
A = εbc
Where:
- A represents the absorbance of the solution (unitless). Absorbance is a logarithmic measure of the transmitted light's intensity.
- ε represents the molar absorptivity (or molar extinction coefficient) (L mol⁻¹ cm⁻¹). This is a constant that is specific to the analyte and the wavelength of light used. It represents how strongly the analyte absorbs light at a particular wavelength. A higher molar absorptivity indicates stronger absorption.
- b represents the path length of the light through the solution (cm). This is the distance the light travels through the sample, usually the width of the cuvette used in the spectrophotometer.
- c represents the concentration of the analyte (mol L⁻¹). This is the amount of analyte present in the solution.
The Significance of Molar Absorptivity (ε)
The molar absorptivity (ε) is a crucial parameter in the Beer-Lambert Law. It’s a measure of how effectively a substance absorbs light at a specific wavelength. Different substances have unique molar absorptivities, and even the same substance can exhibit different molar absorptivities at different wavelengths. This property allows for the selective measurement of specific analytes in a mixture, providing the basis for many quantitative analytical techniques. The wavelength at which the molar absorptivity is highest is often chosen for analysis to maximize sensitivity.
Path Length (b) and its Importance
The path length (b) is the distance the light beam travels through the sample solution. It’s typically the internal width of the cuvette or sample cell used in the spectrophotometer. Maintaining a consistent path length is crucial for accurate measurements. Any variation in path length will directly affect the absorbance reading, thus impacting the calculated concentration. Standardized cuvettes with a precise path length (often 1 cm) are used to ensure consistent and accurate results.
Absorbance vs. Transmittance
It’s important to differentiate between absorbance (A) and transmittance (T). Transmittance is the fraction of incident light that passes through the sample without being absorbed. It is usually expressed as a percentage (%T). The relationship between absorbance and transmittance is logarithmic:
A = -log₁₀T or T = 10⁻ᴬ
Therefore, a higher absorbance corresponds to lower transmittance, indicating stronger absorption of light by the sample.
Factors Affecting Absorbance
Several factors can influence the absorbance of a solution besides concentration and path length, leading to deviations from the Beer-Lambert Law. These factors must be carefully considered to obtain accurate and reliable results.
1. Wavelength of Light
The molar absorptivity (ε) is highly dependent on the wavelength of light. The absorbance of a substance will vary significantly across different wavelengths. A spectrophotometer allows selection of a specific wavelength, typically at the wavelength of maximum absorption (λmax), where the sensitivity of the measurement is highest. Choosing the incorrect wavelength can lead to significant errors.
2. Temperature
Temperature changes can affect the analyte's concentration and its ability to absorb light. Temperature variations can influence the equilibrium of reactions involving the analyte, thereby altering its concentration. Furthermore, changes in temperature can affect the solvent's properties, potentially impacting the analyte's interaction with light. Therefore, maintaining a constant temperature is crucial for accurate measurements.
3. Solvent Effects
The solvent used can significantly impact the analyte's absorption properties. Solvent molecules can interact with the analyte, influencing its electronic structure and thus its ability to absorb light. The refractive index of the solvent also plays a role, affecting the propagation of light through the solution. Therefore, using the same solvent for both standards and samples is critical for obtaining accurate and consistent results.
4. Chemical Interactions
Chemical interactions between the analyte and other components in the solution can also affect absorbance. Reactions such as complex formation, dimerization, or aggregation can alter the analyte's absorption characteristics. These interactions can cause deviations from the linear relationship predicted by the Beer-Lambert Law.
5. Stray Light
Stray light refers to light that reaches the detector without passing through the sample. This light can lead to underestimation of absorbance, especially at high concentrations. This is because the stray light adds to the detected light intensity, making the absorbance appear lower than the actual value. High-quality spectrophotometers minimize stray light to ensure accuracy.
Deviations from the Beer-Lambert Law
While the Beer-Lambert Law provides a good approximation of the relationship between absorbance and concentration under ideal conditions, deviations can occur under certain circumstances. These deviations can be categorized as either chemical or instrumental.
Chemical Deviations
- Chemical interactions: As mentioned earlier, chemical reactions involving the analyte, such as dimerization or complex formation, can cause deviations from linearity.
- Non-ideal solutions: High analyte concentrations can lead to interactions between analyte molecules, affecting their absorption properties. This is often observed at concentrations above 0.01 M.
- Changes in chemical environment: Changes in pH, ionic strength, or solvent composition can alter the analyte's chemical state and thus its absorbance.
Instrumental Deviations
- Stray light: As discussed, stray light reaching the detector without passing through the sample can cause underestimation of absorbance.
- Non-monochromatic light: If the light source is not monochromatic (i.e., contains a range of wavelengths), the absorbance measurement will be less accurate since ε varies with wavelength.
- Improper cuvette: Scratches or fingerprints on the cuvette can scatter light, leading to inaccurate absorbance measurements. Mismatched cuvettes can introduce path length differences.
Applications of the Beer-Lambert Law
The Beer-Lambert Law finds widespread application in various fields, including:
1. Quantitative Analysis
This is the most common application. By measuring the absorbance of a solution of known concentration (standard solution), the molar absorptivity (ε) can be determined. This value can then be used to calculate the concentration of an unknown sample with the same analyte by measuring its absorbance. This technique is used extensively in environmental monitoring, clinical diagnostics, and pharmaceutical analysis.
2. Spectrophotometry
Spectrophotometry is a technique that uses the Beer-Lambert Law to measure the absorbance of a solution at different wavelengths. A spectrophotometer measures the absorbance spectrum, a plot of absorbance versus wavelength, providing information about the analyte's identity and concentration. This is a powerful tool in qualitative and quantitative analysis.
3. Kinetic Studies
The Beer-Lambert Law can be used to monitor the progress of a chemical reaction. By measuring the absorbance of the reaction mixture over time, the concentration of reactants or products can be determined, allowing the determination of reaction rates and mechanisms.
4. Environmental Monitoring
The Beer-Lambert Law is employed in various environmental monitoring applications, such as determining the concentration of pollutants in water or air samples. Specific analytes can be identified and quantified using appropriate wavelengths and standards.
5. Clinical Diagnostics
In clinical laboratories, the Beer-Lambert Law is used in blood analysis to measure the concentrations of various components, such as glucose, proteins, and enzymes. These measurements are crucial for diagnosing and monitoring various medical conditions.
Conclusion
The Beer-Lambert Law provides a powerful and fundamental relationship between absorbance and concentration. While deviations from this law can occur, understanding these limitations and implementing proper experimental techniques are crucial for obtaining accurate and reliable results. Its wide-ranging applications across diverse scientific disciplines highlight its importance as a cornerstone principle in analytical chemistry and spectroscopy. By mastering the principles of the Beer-Lambert Law, scientists and researchers can perform precise quantitative analyses, facilitating advancements in numerous fields. Careful attention to experimental details, including wavelength selection, temperature control, solvent selection, and instrument calibration, ensures accurate results and facilitates reliable interpretation of data obtained using this essential scientific tool.
Latest Posts
Latest Posts
-
Label The Cavities Of The Head
Mar 27, 2025
-
What Is A Stable Electron Configuration
Mar 27, 2025
-
What Is A Joto In Spanish
Mar 27, 2025
-
Perceptual Motor Skills And Movement Concepts
Mar 27, 2025
-
Do Homologous Chromosomes Pair In Mitosis
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between Absorbance And Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
