What Is A Stable Electron Configuration
Muz Play
Mar 27, 2025 · 6 min read
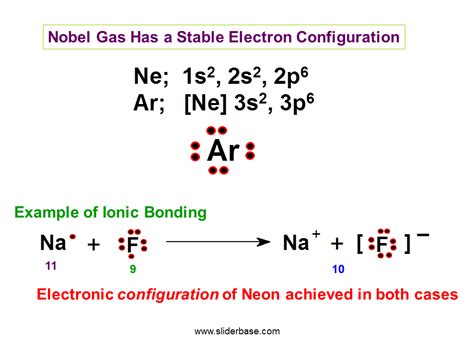
Table of Contents
What is a Stable Electron Configuration? Achieving Atomic Harmony
The quest for stability is a universal theme, evident from the smallest atoms to the largest galaxies. In the realm of chemistry, this drive for stability manifests most profoundly in the electron configuration of atoms. Understanding what constitutes a stable electron configuration is fundamental to grasping the reactivity of elements and the formation of chemical bonds. This article delves deep into this fascinating aspect of atomic structure, exploring the reasons behind stability, the rules governing electron arrangement, and the implications for chemical behavior.
The Octet Rule: A Cornerstone of Stability
The most commonly cited principle related to stable electron configurations is the octet rule. This rule postulates that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell containing eight electrons, similar to the electron configuration of noble gases. Noble gases, located in Group 18 of the periodic table (Helium, Neon, Argon, Krypton, Xenon, Radon), are exceptionally unreactive because their outermost electron shell is completely filled. This filled shell represents a state of maximum stability.
Exceptions to the Octet Rule
While the octet rule serves as a useful guideline, it's crucial to acknowledge its exceptions. Some elements, particularly those in the third period and beyond, can accommodate more than eight electrons in their valence shell due to the availability of empty d orbitals. Examples include phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆). Additionally, certain atoms, especially those with low atomic numbers like hydrogen and lithium, are stable with only two electrons in their outer shell (a duet). This is because their outermost shell is the first shell, which only has a capacity of two electrons (in the 1s orbital).
Electron Shells and Subshells: The Building Blocks of Configuration
To understand stable electron configurations, it’s essential to grasp the concept of electron shells and subshells. Electrons occupy specific energy levels within an atom, organized into shells and subshells.
-
Electron Shells (Principal Energy Levels): These represent the average distance of an electron from the nucleus. They are designated by principal quantum numbers (n), where n = 1, 2, 3, etc. Shells closer to the nucleus have lower energy levels.
-
Electron Subshells (Sublevels): Within each shell, electrons are further categorized into subshells, distinguished by their shapes and energy levels. These are designated by letters: s, p, d, and f. Each subshell has a specific number of orbitals:
- s subshell: 1 orbital (holds up to 2 electrons)
- p subshell: 3 orbitals (holds up to 6 electrons)
- d subshell: 5 orbitals (holds up to 10 electrons)
- f subshell: 7 orbitals (holds up to 14 electrons)
Filling Order and the Aufbau Principle
The Aufbau principle dictates the order in which electrons fill the subshells: electrons first fill the lowest energy levels available. This leads to a predictable filling pattern, often visualized using the Aufbau diagram or a periodic table. However, exceptions exist, particularly for transition metals and lanthanides/actinides, where electron-electron repulsions can influence the filling order slightly.
Hund's Rule and the Pauli Exclusion Principle: Shaping Electron Arrangements
Two additional principles govern the arrangement of electrons within subshells:
-
Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion. Each electron in a singly occupied orbital has the same spin (either spin up or spin down).
-
Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers (n, l, ml, ms). This means each orbital can hold a maximum of two electrons, with opposite spins.
Stable Configurations and Chemical Reactivity
The stability of an electron configuration directly influences an atom's chemical reactivity. Atoms with unstable configurations, meaning their outer shells are not completely filled, tend to react with other atoms to achieve a more stable configuration. This is often achieved through the formation of chemical bonds:
-
Ionic Bonds: Atoms transfer electrons to achieve a stable octet (or duet). This creates ions with opposite charges that attract each other, forming an ionic compound. For example, sodium (Na) readily loses one electron to become Na⁺, while chlorine (Cl) readily gains one electron to become Cl⁻. The electrostatic attraction between Na⁺ and Cl⁻ forms sodium chloride (NaCl).
-
Covalent Bonds: Atoms share electrons to achieve stable octets. This sharing creates a covalent bond, as seen in molecules like methane (CH₄) and water (H₂O).
Beyond the Octet: Expanded Octet and Incomplete Octet
As mentioned earlier, the octet rule has exceptions. Elements in the third period and beyond can exhibit expanded octets, meaning they can have more than eight electrons in their valence shell. This is possible because of the involvement of their empty d orbitals which can accommodate additional electrons. The participation of d orbitals in bonding allows for higher coordination numbers and the formation of molecules like phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆).
Conversely, some atoms, particularly those with low atomic numbers, may exhibit an incomplete octet, possessing fewer than eight electrons in their outer shell. Boron trifluoride (BF₃) is a classic example where boron only has six valence electrons. The driving force for stability in these cases involves maximizing bond formation to gain energy.
Predicting Electron Configurations: A Practical Approach
Predicting the electron configuration of an atom involves understanding the filling order of subshells and applying the Aufbau principle, Hund's rule, and the Pauli exclusion principle. Let's consider an example: oxygen (O, atomic number 8).
- Oxygen has 8 electrons.
- The filling order is 1s, 2s, 2p.
- The 1s subshell fills first with 2 electrons (1s²).
- The 2s subshell fills next with 2 electrons (2s²).
- The remaining 4 electrons fill the 2p subshell. According to Hund's rule, these electrons occupy each 2p orbital individually before pairing up (2p⁴).
Therefore, the complete electron configuration of oxygen is 1s²2s²2p⁴. This configuration is unstable because the 2p subshell is not completely filled. Oxygen readily forms covalent bonds to achieve a more stable octet.
Stable Configurations and Periodic Trends
The stability of electron configurations significantly impacts periodic trends. For instance, ionization energy, electron affinity, and electronegativity are all influenced by how close an atom is to achieving a stable electron configuration. Atoms that are close to achieving a noble gas configuration will tend to have higher ionization energies (requiring more energy to remove an electron) and higher electron affinities (a greater tendency to gain an electron). Electronegativity, the ability of an atom to attract electrons in a bond, is also higher for atoms close to achieving a stable configuration.
Conclusion: The Pursuit of Atomic Stability
The pursuit of a stable electron configuration is a fundamental driving force in chemistry. Understanding the octet rule, along with exceptions like expanded and incomplete octets, allows for a deeper understanding of the chemical behavior of elements. The interplay between electron shells, subshells, and governing principles like Hund's rule and the Pauli exclusion principle dictates the arrangement and stability of electrons, ultimately shaping the reactivity and bonding properties of atoms. By mastering these concepts, we can unlock a comprehensive understanding of the atomic world and its intricate relationships. This knowledge forms the foundation for exploring a wide range of chemical phenomena and applications, from the formation of simple molecules to the complex reactions underpinning life itself.
Latest Posts
Latest Posts
-
Why Is Rusting A Chemical Change
Mar 30, 2025
-
Difference Between Dipole Dipole And London Dispersion Forces
Mar 30, 2025
-
Which Is The Most Reactive Element
Mar 30, 2025
-
Personal Pronouns Have Number Person And What
Mar 30, 2025
-
Is Density And Specific Gravity The Same
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is A Stable Electron Configuration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
