Table Of Standard Heats Of Formation
Muz Play
Mar 24, 2025 · 6 min read
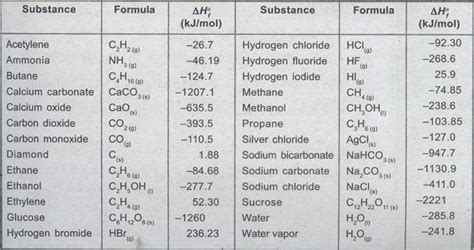
Table of Contents
Understanding and Utilizing the Table of Standard Heats of Formation
The table of standard heats of formation, also known as the table of standard enthalpies of formation, is a crucial resource in chemistry and related fields. It provides a fundamental understanding of chemical reactions and energy changes. This comprehensive guide will delve into the intricacies of this table, explaining its construction, application, and significance in various calculations. We will explore its uses in determining reaction enthalpies, understanding spontaneity, and predicting reaction feasibility.
What is Standard Heat of Formation?
The standard heat of formation (ΔHf°), or standard enthalpy of formation, is the change in enthalpy that accompanies the formation of one mole of a substance in its standard state from its constituent elements in their standard states, with all substances at a standard temperature and pressure (usually 298.15 K and 1 atm). It's a key thermodynamic property that reflects the stability of a compound. A negative ΔHf° indicates that the formation of the compound is exothermic (releases heat), while a positive ΔHf° signifies an endothermic process (absorbs heat). The standard state of an element is its most stable form under standard conditions. For example, the standard state of oxygen is O₂(g), not O(g).
Key Concepts to Understand:
- Standard State: The most stable physical state of a substance at 1 atm pressure and a specified temperature (usually 25°C or 298.15 K).
- Enthalpy (H): A thermodynamic function representing the total heat content of a system. Changes in enthalpy (ΔH) reflect the heat absorbed or released during a process at constant pressure.
- Exothermic Reaction: A reaction that releases heat to its surroundings (ΔH < 0).
- Endothermic Reaction: A reaction that absorbs heat from its surroundings (ΔH > 0).
Constructing the Table of Standard Heats of Formation
The values in the table of standard heats of formation are experimentally determined, often through calorimetry. Precise measurements are crucial because small errors can accumulate and significantly affect calculations involving multiple steps. The data is meticulously compiled and verified, ensuring accuracy and reliability. The table usually includes:
- Compound: The chemical formula of the substance.
- Phase: The physical state of the substance (solid (s), liquid (l), gas (g), aqueous (aq)). The phase is critical because the enthalpy of formation varies with the phase.
- ΔHf° (kJ/mol): The standard heat of formation in kilojoules per mole.
Utilizing the Table: Calculating Reaction Enthalpies
The primary application of the table of standard heats of formation is in calculating the standard enthalpy change (ΔH°rxn) of a chemical reaction. This is done using Hess's Law, which states that the enthalpy change of a reaction is independent of the pathway taken. The calculation utilizes the following equation:
ΔH°rxn = Σ [ΔHf°(products)] - Σ [ΔHf°(reactants)]
This equation means that the standard enthalpy change of a reaction is the sum of the standard heats of formation of the products minus the sum of the standard heats of formation of the reactants. Each ΔHf° value is multiplied by the stoichiometric coefficient of the corresponding substance in the balanced chemical equation.
Example Calculation:
Consider the combustion of methane:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
To calculate ΔH°rxn, we would use the standard heats of formation from the table:
- ΔHf°[CH₄(g)]
- ΔHf°[O₂(g)] = 0 (since it's an element in its standard state)
- ΔHf°[CO₂(g)]
- ΔHf°[H₂O(l)]
Substituting these values into the equation above would yield the standard enthalpy change for the combustion of methane.
Beyond Reaction Enthalpies: Other Applications
The table of standard heats of formation extends beyond simply calculating reaction enthalpies. It's a versatile tool with applications in various areas:
1. Predicting Reaction Spontaneity:
While the enthalpy change (ΔH) provides information about the heat transfer during a reaction, it doesn't fully determine spontaneity. Spontaneity is also influenced by the entropy change (ΔS), a measure of disorder. The Gibbs Free Energy (ΔG) combines both enthalpy and entropy to predict spontaneity:
ΔG = ΔH - TΔS
A negative ΔG indicates a spontaneous reaction, while a positive ΔG suggests a non-spontaneous reaction. The table of standard heats of formation provides the ΔH component of this calculation, allowing for a more complete assessment of reaction spontaneity.
2. Assessing Reaction Feasibility:
The standard enthalpy change (ΔH°rxn) helps to evaluate the feasibility of a reaction. A highly negative ΔH°rxn generally suggests a reaction is more likely to occur, although other factors, like activation energy and kinetics, also play important roles.
3. Understanding Bond Energies:
While not a direct application, the data in the table can be used in conjunction with bond energies to estimate the strength of chemical bonds and understand the energetic factors influencing molecular stability.
4. Industrial Process Optimization:
In industrial chemistry, the table helps optimize reaction conditions, select catalysts, and improve process efficiency by predicting energy requirements and potential for unwanted side reactions.
Limitations and Considerations
While extremely useful, the table of standard heats of formation has limitations:
- Standard Conditions: The values are specific to standard conditions (298.15 K and 1 atm). Deviations from these conditions will affect the actual enthalpy change.
- Ideal Behavior: The values assume ideal behavior of gases and solutions. Deviations from ideality can introduce errors.
- Accuracy of Data: While carefully measured, experimental uncertainties exist in the reported values. These uncertainties propagate through calculations.
- Limited Scope: The table may not include all substances, particularly less common or newly synthesized compounds.
Accessing and Using the Table
The table of standard heats of formation is readily available in various chemistry textbooks, handbooks, and online databases. Many online resources provide searchable tables, allowing for convenient access to the data. When using the table, always ensure that you are using a reliable and up-to-date source. Pay close attention to the units (kJ/mol) and the specified temperature and pressure.
Conclusion
The table of standard heats of formation is an invaluable tool for chemists and related professionals. Its application in calculating reaction enthalpies, assessing reaction spontaneity and feasibility, and understanding reaction energetics is indispensable. While limitations exist, understanding these limitations and using the table responsibly ensures accurate and meaningful interpretations of thermodynamic data. By combining this knowledge with other thermodynamic concepts, a comprehensive understanding of chemical reactions and their energy changes can be achieved. Through meticulous attention to detail and a thorough understanding of the underlying principles, the table serves as a powerful instrument in predicting and analyzing chemical processes. Further research into more advanced thermodynamic concepts will build upon the foundation provided by this essential table, allowing for increasingly precise and insightful analysis of chemical systems.
Latest Posts
Latest Posts
-
Will Acidic And Basic Solutions React The Same On Skin
Mar 27, 2025
-
Isothermal Expansion Of An Ideal Gas
Mar 27, 2025
-
Which Types Of Viruses Are Released By Budding
Mar 27, 2025
-
Is Fungi A Eukaryote Or Prokaryote
Mar 27, 2025
-
Slope In Position Vs Time Graph
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Table Of Standard Heats Of Formation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
