Isothermal Expansion Of An Ideal Gas
Muz Play
Mar 27, 2025 · 6 min read
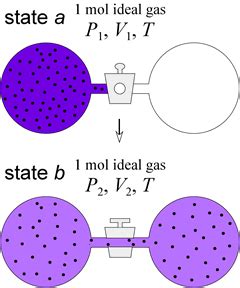
Table of Contents
Isothermal Expansion of an Ideal Gas: A Comprehensive Guide
Isothermal expansion, a fundamental concept in thermodynamics, describes the process where an ideal gas expands at a constant temperature. Understanding this process is crucial for grasping various thermodynamic principles and their applications in diverse fields like engineering, chemistry, and physics. This comprehensive guide delves into the intricacies of isothermal expansion, exploring its underlying principles, equations, calculations, and real-world applications.
Understanding Isothermal Processes
Before diving into isothermal expansion specifically, let's establish a clear understanding of isothermal processes in general. An isothermal process is any thermodynamic process that occurs at a constant temperature. This implies that there's no temperature change (ΔT = 0) during the process. Maintaining a constant temperature often requires a slow expansion or contraction and contact with a large thermal reservoir, ensuring heat can flow freely into or out of the system to compensate for any internal energy changes. This heat exchange is key to understanding why the temperature remains constant.
Ideal Gas Law and its Role
The behavior of an ideal gas during isothermal expansion is primarily governed by the ideal gas law:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R represents the ideal gas constant
- T represents temperature (in Kelvin)
Since the temperature (T) remains constant during an isothermal process, the ideal gas law simplifies the relationship between pressure and volume. If we rearrange the equation for an isothermal process, we get:
PV = constant
This crucial relationship highlights the inverse proportionality between pressure and volume at a constant temperature. As the volume increases, the pressure decreases proportionally, and vice-versa. This inverse relationship is visualized graphically as a hyperbola on a PV diagram.
Isothermal Expansion: A Detailed Exploration
Now let's focus specifically on isothermal expansion. This is a scenario where an ideal gas expands while its temperature is held constant. Several factors can drive isothermal expansion, including:
-
External pressure reduction: If the external pressure acting on a gas is lowered, the gas will expand to equalize the pressure difference. This process can be slow enough to allow heat transfer to maintain a constant temperature.
-
Heat input: If heat is supplied to the gas during the expansion, it can counterbalance the work done by the gas and maintain a constant temperature. This is often achieved using a thermal reservoir.
Work Done During Isothermal Expansion
One of the most significant aspects of isothermal expansion is calculating the work done by the gas during the expansion. Since the pressure isn't constant throughout the expansion (as we learned from PV=constant), a simple multiplication of pressure and change in volume isn't sufficient. Instead, we use calculus to integrate the pressure-volume relationship over the change in volume:
W = ∫PdV
Substituting the ideal gas law, P = nRT/V:
W = ∫(nRT/V)dV
Integrating this from the initial volume (Vᵢ) to the final volume (V<sub>f</sub>) gives us:
W = nRT ln(V<sub>f</sub>/Vᵢ)
This equation reveals that the work done during isothermal expansion depends on the number of moles (n), the ideal gas constant (R), the temperature (T), and the ratio of the final and initial volumes (V<sub>f</sub>/Vᵢ). Notice that the work done is directly proportional to the temperature and the natural logarithm of the volume ratio. The larger the volume expansion, the larger the work done. A positive value for W indicates that the gas performs work on its surroundings (expansion).
Heat Transfer During Isothermal Expansion
As mentioned previously, maintaining a constant temperature during expansion requires heat transfer. For an isothermal expansion, the heat (Q) absorbed by the gas exactly equals the work (W) done by the gas. This is a direct consequence of the first law of thermodynamics for this specific process:
ΔU = Q - W
Since ΔU (change in internal energy) is zero for a constant temperature process (isothermal), we have:
Q = W = nRT ln(V<sub>f</sub>/Vᵢ)
This equation shows the crucial relationship between heat transfer and work in an isothermal expansion. The heat absorbed by the gas is used entirely to perform the work of expansion. Without heat input, the gas would cool as it expands (adiabatic expansion).
Isothermal Expansion vs. Other Expansion Processes
Comparing isothermal expansion with other types of expansion processes is crucial for a thorough understanding of its unique characteristics. Here are two key comparisons:
Isothermal Expansion vs. Adiabatic Expansion
Adiabatic expansion is a process where no heat exchange occurs with the surroundings (Q = 0). This leads to a decrease in temperature during the expansion as the gas performs work. Unlike isothermal expansion where temperature is constant, the temperature of the gas changes significantly during adiabatic expansion. The work done and pressure-volume relationship differ significantly as well. Adiabatic processes are usually characterized by rapid changes, preventing significant heat exchange.
Isothermal Expansion vs. Isobaric Expansion
Isobaric expansion occurs at constant pressure (ΔP = 0). This contrasts with isothermal expansion, where pressure changes inversely with volume. In an isobaric process, the work done is simpler to calculate (W = PΔV). Isobaric expansion might involve controlled heating to maintain the constant pressure while the volume increases.
Applications of Isothermal Expansion
Isothermal expansion, despite its seemingly theoretical nature, finds numerous applications in various real-world scenarios. These include:
-
Refrigeration and Air Conditioning: The expansion of refrigerants in the expansion valve of a refrigeration cycle approximates an isothermal process, contributing to the cooling effect. The expansion leads to a decrease in refrigerant temperature due to the work done against external pressure.
-
Internal Combustion Engines: While not strictly isothermal, certain stages of internal combustion engine cycles, especially the expansion stroke, can be modeled as an approximate isothermal process, helping engineers analyze engine performance.
-
Chemical Processes: Many chemical reactions and processes occur at controlled temperatures. Understanding isothermal expansion is essential for designing and optimizing these processes.
-
Meteorology: Understanding atmospheric processes, including the expansion of air masses, often involves applying isothermal principles as approximations.
-
Biological Systems: Certain biological processes involve gas exchange, which could be approximated using isothermal expansion principles.
Illustrative Example: Calculating Work Done in Isothermal Expansion
Let's consider a concrete example to illustrate the calculation of work done during isothermal expansion. Suppose we have 2 moles of an ideal gas at a temperature of 300 K and an initial volume of 5 liters. The gas expands isothermally to a final volume of 10 liters. We can calculate the work done using the formula:
W = nRT ln(V<sub>f</sub>/Vᵢ)
Plugging in the values (using R = 8.314 J/mol·K):
W = (2 mol)(8.314 J/mol·K)(300 K) ln(10 L / 5 L)
W ≈ 3457 J
This calculation demonstrates how to apply the isothermal expansion equation to determine the work done by the gas.
Conclusion
Isothermal expansion is a critical concept in thermodynamics with wide-ranging applications. Understanding the relationship between pressure, volume, work, and heat transfer at a constant temperature is essential for analyzing and predicting the behavior of ideal gases in various real-world systems. From refrigeration to chemical processes and even atmospheric phenomena, the principles of isothermal expansion provide a valuable framework for comprehension and application. While the assumption of an ideal gas might represent a simplification in some cases, its utility in approximating real-world behavior makes it a cornerstone concept in the study of thermodynamics. The detailed analysis presented here aims to equip readers with a comprehensive understanding of this vital thermodynamic process.
Latest Posts
Latest Posts
-
How Are The Nervous System And Endocrine System Similar
Mar 30, 2025
-
What Is The Function Of Atrioventricular Valves
Mar 30, 2025
-
The Tape Diagram Represents An Equation
Mar 30, 2025
-
Lewis Structure For Co With Formal Charges
Mar 30, 2025
-
A Solution Is A Homogeneous Mixture
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Isothermal Expansion Of An Ideal Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
