Lewis Structure For Co With Formal Charges
Muz Play
Mar 30, 2025 · 6 min read
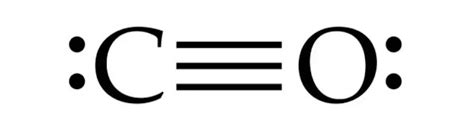
Table of Contents
Lewis Structure for CO with Formal Charges: A Comprehensive Guide
Carbon monoxide (CO), a simple yet fascinating molecule, presents a unique challenge when it comes to drawing its Lewis structure and assigning formal charges. While seemingly straightforward, understanding the nuances of its bonding and the implications of formal charges is crucial for grasping fundamental concepts in chemistry. This comprehensive guide will delve into the intricacies of the CO Lewis structure, exploring different approaches, explaining formal charge calculations, and clarifying potential misconceptions.
Understanding the Basics: Lewis Structures and Formal Charges
Before diving into the specifics of CO, let's refresh our understanding of Lewis structures and formal charges.
Lewis Structures: These diagrams represent the valence electrons of atoms within a molecule, illustrating how atoms bond and share electrons to achieve a stable electron configuration, typically following the octet rule (eight valence electrons). They show single, double, and triple bonds, as well as lone pairs of electrons.
Formal Charges: Formal charges are hypothetical charges assigned to atoms in a Lewis structure. They help determine the most plausible structure when multiple valid structures are possible. A formal charge is calculated as:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 Bonding Electrons)
A lower magnitude of formal charges overall usually indicates a more stable structure. Ideally, the sum of formal charges in a neutral molecule should equal zero.
Constructing the Lewis Structure of CO
Carbon (C) has four valence electrons, and oxygen (O) has six. Therefore, the total number of valence electrons in CO is 10. Let's explore different possible structures:
Option 1: Single Bond
If we attempt a single bond between C and O, we get:
:C-O:
This leaves 8 electrons to be distributed. Oxygen can accommodate these as lone pairs, but carbon only has 6 electrons in its valence shell, failing to satisfy the octet rule. This structure is highly unstable.
Option 2: Double Bond
Let's try a double bond:
:C=O:
This structure still leaves 6 electrons unaccounted for. Again, oxygen can accommodate these lone pairs, but carbon is short two electrons. While better than the single bond option, it also doesn't satisfy the octet rule. It is also unstable.
Option 3: Triple Bond
Finally, let's consider a triple bond:
:C≡O:
This structure uses all 10 valence electrons. Carbon and oxygen both have a complete octet (8 electrons). This structure is the most stable representation of CO.
Calculating Formal Charges for the Triple Bond Structure
Let's calculate the formal charges for the most stable structure (triple bond):
For Carbon (C):
- Valence electrons = 4
- Non-bonding electrons = 2 (one lone pair)
- Bonding electrons = 6 (triple bond)
- Formal Charge = 4 - 2 - (6/2) = 0
For Oxygen (O):
- Valence electrons = 6
- Non-bonding electrons = 2 (one lone pair)
- Bonding electrons = 6 (triple bond)
- Formal Charge = 6 - 2 - (6/2) = 2
The oxygen atom carries a formal charge of +2 and the carbon atom carries a formal charge of 0. This seems unusual; however, it is crucial to note that formal charge is a calculated value and does not necessarily represent the actual charge distribution in the molecule.
Understanding the Discrepancy in Formal Charges
The seemingly unusual formal charges in the triple-bonded structure highlight a limitation of the formal charge concept. While useful, it's a simplification. Electronegativity plays a crucial role in determining the real charge distribution. Oxygen is significantly more electronegative than carbon, meaning it attracts electrons more strongly.
In reality, the bonding electrons are not equally shared; the electron density is shifted towards the more electronegative oxygen atom. This leads to a polar bond, with oxygen having a partial negative charge (δ-) and carbon having a partial positive charge (δ+). This polar nature of the CO bond influences its chemical reactivity.
Resonance Structures in CO
While the triple bond structure is the most stable, we can explore the concept of resonance to further refine our understanding. Resonance structures are different Lewis structures that can be drawn for the same molecule, where only the placement of electrons differs. For CO, while not as significant as in other molecules, we can consider a resonance structure with a quadruple bond (although this is a less commonly discussed aspect):
This structure is less favored due to the high energy cost associated with this bond order.
Applications and Importance of Understanding CO's Structure
Understanding the Lewis structure and the implications of formal charges in CO is vital in several areas:
- Predicting Reactivity: The polar nature of the CO bond, stemming from the electronegativity difference and the distribution of electron density, determines how CO interacts with other molecules. This understanding is crucial in fields like catalysis and organic chemistry.
- Spectroscopy: The structure of CO directly influences its vibrational and rotational frequencies, observable through spectroscopic techniques like infrared (IR) and Raman spectroscopy.
- Inorganic Chemistry: CO is a crucial ligand in many metal complexes, forming strong metal-carbon bonds. Understanding its electronic structure is essential for comprehending the properties and reactivity of these complexes.
- Biochemistry: CO plays a role in certain biological processes, interacting with heme proteins. Understanding its bonding is necessary for studying its interactions with biological systems.
- Industrial Applications: CO is a significant industrial gas used in various processes, including the production of methanol and other chemicals. Understanding its properties is crucial for safe and efficient handling.
Addressing Common Misconceptions
- Formal charge equals actual charge: This is a critical misconception. Formal charge is a calculated value; it doesn't represent the actual charge distribution within the molecule.
- Octet rule is absolute: While the octet rule serves as a useful guideline, there are exceptions. CO is one such example where the octet rule is not strictly followed by carbon, though it has an expanded valence shell.
- Only one Lewis structure is correct: Multiple resonance structures can be drawn for a single molecule, each contributing to the overall picture of bonding.
Conclusion
The Lewis structure of carbon monoxide, particularly the assignment of formal charges, offers a valuable lesson in understanding chemical bonding and the limitations of simplified models. While the triple bond structure provides the most stable representation, considering formal charges and electronegativity provides a more complete and accurate picture of the molecule's electronic structure. This understanding is crucial for predicting reactivity, interpreting spectroscopic data, and exploring applications of CO across various scientific disciplines. By grasping the nuances of CO's bonding, we gain a deeper understanding of chemical bonding principles applicable to many other molecules. The apparent discrepancy in formal charges underscores the importance of considering electronegativity and the actual distribution of electron density in a molecule rather than relying solely on formal charge calculations. This case study perfectly demonstrates that the seemingly simple concept of Lewis structures and formal charges requires a thorough understanding of multiple underlying chemical principles.
Latest Posts
Latest Posts
-
What Is The Difference Between Self Esteem And Self Efficacy
Apr 01, 2025
-
Classify Each Of The Substances As An Element Or Compound
Apr 01, 2025
-
The Atomic Radii Of The Elements In The Nitrogen Group
Apr 01, 2025
-
What Is The Purpose Of Using A Pedigree
Apr 01, 2025
-
How To Tell If A Function Is Continuous Without Graphing
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure For Co With Formal Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
