Organic Chemistry Substitution And Elimination Reactions
Muz Play
Mar 23, 2025 · 6 min read
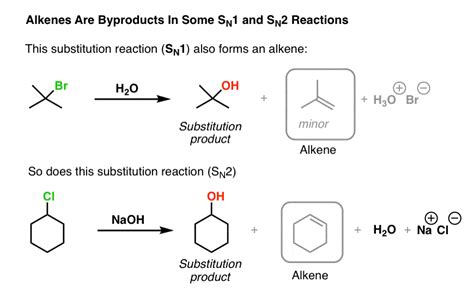
Table of Contents
Organic Chemistry: A Deep Dive into Substitution and Elimination Reactions
Organic chemistry, the study of carbon-containing compounds, is a vast and intricate field. Within this field, substitution and elimination reactions stand as fundamental reaction types, forming the backbone of many synthetic strategies and appearing frequently in biochemical processes. Understanding these reactions is crucial for any aspiring chemist. This comprehensive guide will delve into the intricacies of substitution and elimination reactions, covering their mechanisms, factors influencing their occurrence, and practical applications.
Understanding Substitution Reactions
Substitution reactions, as the name suggests, involve the replacement of one atom or group in a molecule with another. This exchange typically occurs at a saturated carbon atom (a carbon atom with four single bonds). There are two primary mechanistic pathways for substitution reactions: SN1 and SN2.
SN1 Reactions (Unimolecular Nucleophilic Substitution)
SN1 reactions are first-order reactions, meaning their rate depends only on the concentration of the substrate. This implies a two-step mechanism:
Step 1: Ionization
The leaving group departs from the substrate, forming a carbocation intermediate. This step is the rate-determining step, and its speed is influenced by the stability of the carbocation. Tertiary carbocations are the most stable, followed by secondary, and then primary carbocations. Methyl carbocations are the least stable.
Step 2: Nucleophilic Attack
The nucleophile attacks the carbocation, forming a new bond and completing the substitution. This step is generally fast.
Factors influencing SN1 reactions:
- Substrate structure: Tertiary substrates > secondary substrates > primary substrates (due to carbocation stability).
- Leaving group ability: Good leaving groups (e.g., I⁻, Br⁻, Cl⁻, TsO⁻) facilitate the ionization step. Weaker bases are better leaving groups.
- Solvent: Polar protic solvents (e.g., water, alcohols) stabilize both the carbocation and the leaving group, enhancing the reaction rate.
- Nucleophile strength: The nucleophile's strength is less important in SN1 reactions since it doesn't participate in the rate-determining step.
SN2 Reactions (Bimolecular Nucleophilic Substitution)
SN2 reactions are second-order reactions, meaning their rate depends on the concentration of both the substrate and the nucleophile. This indicates a single-step, concerted mechanism:
The nucleophile attacks the substrate from the backside, while simultaneously the leaving group departs. This results in inversion of configuration at the stereocenter.
Factors influencing SN2 reactions:
- Substrate structure: Methyl substrates > primary substrates > secondary substrates (steric hindrance significantly impacts the reaction rate; tertiary substrates generally do not undergo SN2 reactions).
- Leaving group ability: Similar to SN1, good leaving groups are favored.
- Nucleophile strength: Strong nucleophiles (e.g., HO⁻, RO⁻, CN⁻, RS⁻) are essential for SN2 reactions. Their concentration directly affects the rate.
- Solvent: Polar aprotic solvents (e.g., DMSO, DMF, acetone) are preferred as they solvate the cation, leaving the nucleophile less solvated and therefore more reactive.
Comparison of SN1 and SN2 Reactions:
| Feature | SN1 | SN2 |
|---|---|---|
| Mechanism | Two-step | One-step |
| Rate law | Rate = k[substrate] | Rate = k[substrate][nucleophile] |
| Stereochemistry | Racemization (often, but not always) | Inversion of configuration |
| Substrate | Tertiary > secondary > primary | Methyl > primary > secondary (tertiary generally unreactive) |
| Leaving group | Good leaving group required | Good leaving group required |
| Nucleophile | Nucleophile strength less important | Strong nucleophile required |
| Solvent | Polar protic | Polar aprotic |
Understanding Elimination Reactions
Elimination reactions involve the removal of atoms or groups from a molecule to form a double or triple bond (unsaturated compound). Common elimination reactions include E1 and E2.
E1 Reactions (Unimolecular Elimination)
E1 reactions are first-order reactions, similar to SN1 reactions. They also proceed through a two-step mechanism:
Step 1: Ionization
The leaving group departs from the substrate, forming a carbocation intermediate – the same as in SN1. This is the rate-determining step.
Step 2: Deprotonation
A base abstracts a proton (H⁺) from a carbon atom adjacent to the carbocation, forming a double bond.
Factors influencing E1 reactions:
- Substrate structure: Tertiary substrates > secondary substrates > primary substrates (due to carbocation stability).
- Leaving group ability: Good leaving groups are essential.
- Solvent: Polar protic solvents stabilize the carbocation intermediate.
- Base strength: A weak base is sufficient as the base doesn't participate in the rate-determining step. However, a sufficiently strong base must be present to abstract the proton in the second step.
E2 Reactions (Bimolecular Elimination)
E2 reactions are second-order reactions, with their rate depending on the concentration of both the substrate and the base. They proceed through a concerted, one-step mechanism:
The base abstracts a proton from a carbon atom adjacent to the carbon bearing the leaving group, while simultaneously the leaving group departs. This leads to the formation of a double bond. The stereochemistry often follows an anti-periplanar arrangement, meaning the proton and leaving group are on opposite sides of the molecule.
Factors influencing E2 reactions:
- Substrate structure: Tertiary substrates > secondary substrates > primary substrates (although primary substrates can react, steric effects become more pronounced with increasing substitution).
- Leaving group ability: Good leaving groups are necessary.
- Base strength: Strong bases (e.g., RO⁻, R₂N⁻) are required to abstract the proton.
- Solvent: Polar aprotic solvents are often preferred.
Comparison of E1 and E2 Reactions:
| Feature | E1 | E2 |
|---|---|---|
| Mechanism | Two-step | One-step |
| Rate law | Rate = k[substrate] | Rate = k[substrate][base] |
| Stereochemistry | Not highly stereospecific | Often anti-periplanar |
| Substrate | Tertiary > secondary > primary | Tertiary > secondary > primary (although primary can react) |
| Leaving group | Good leaving group required | Good leaving group required |
| Base | Weak base sufficient | Strong base required |
| Solvent | Polar protic | Polar aprotic often preferred |
Competition between Substitution and Elimination Reactions
Often, the same substrate can undergo both substitution and elimination reactions under certain conditions. The relative amounts of substitution and elimination products depend on several factors:
- Substrate structure: Tertiary substrates favor elimination (E1), while primary substrates favor substitution (SN2). Secondary substrates can undergo both.
- Nucleophile/Base strength: Strong, bulky bases favor elimination (E2), while strong, less hindered nucleophiles favor substitution (SN2). Weak nucleophiles/bases often lead to substitution (SN1) or elimination (E1).
- Solvent: Polar protic solvents favor SN1 and E1, while polar aprotic solvents favor SN2 and E2.
- Temperature: Higher temperatures generally favor elimination reactions due to their higher activation energy.
Practical Applications
Substitution and elimination reactions are ubiquitous in organic synthesis and biochemistry. Some key applications include:
- Synthesis of pharmaceuticals: Many drugs are synthesized using substitution and elimination reactions to introduce specific functional groups.
- Polymer synthesis: These reactions are crucial in the formation of various polymers.
- Natural product synthesis: Understanding these reactions is vital for synthesizing complex natural products.
- Biochemical pathways: Numerous metabolic processes involve substitution and elimination reactions.
Conclusion
Substitution and elimination reactions are fundamental concepts in organic chemistry. A thorough understanding of their mechanisms, influencing factors, and competitive nature is critical for predicting reaction outcomes and designing efficient synthetic strategies. By mastering these principles, organic chemists can effectively manipulate molecules and create new compounds with desired properties. Further exploration into specific examples and variations of these reactions will enhance one’s understanding and skills in organic synthesis and reaction design. Remember, practice and consistent learning are key to mastering this challenging, yet rewarding, area of chemistry.
Latest Posts
Latest Posts
-
Calculating An Equilibrium Constant From A Partial Equilibrium Composition
Mar 24, 2025
-
Table Of Standard Heats Of Formation
Mar 24, 2025
-
What Are The Units Of Heat
Mar 24, 2025
-
Conservation Of Power In A Circuit
Mar 24, 2025
-
What Is The Relationship Between Absorbance And Concentration
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Organic Chemistry Substitution And Elimination Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
