For Most Substances Solubility What As Temperature
Muz Play
Mar 22, 2025 · 6 min read
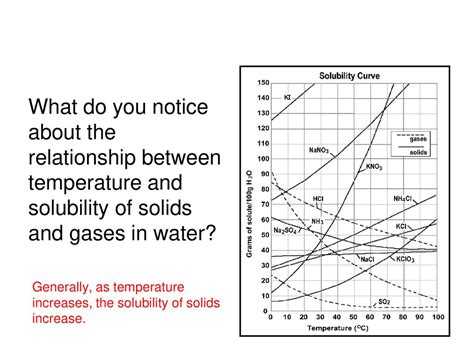
Table of Contents
For Most Substances, Solubility Increases With Temperature: A Deep Dive
The relationship between temperature and solubility is a fundamental concept in chemistry and has significant implications across various fields, from environmental science to pharmaceuticals. While there are exceptions, the general rule is that the solubility of most solids in liquids increases with increasing temperature. This article will delve into the intricacies of this relationship, exploring the underlying principles, exceptions to the rule, and practical applications.
Understanding Solubility
Before exploring the temperature dependence, let's define solubility. Solubility refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure to form a saturated solution. This is usually expressed in units like grams of solute per 100 grams of solvent (g/100g) or moles of solute per liter of solvent (mol/L). The solubility of a substance is determined by the interplay of several factors, including the nature of the solute and solvent, temperature, and pressure.
The Temperature-Solubility Relationship: Why Does It Happen?
The increase in solubility with temperature for most solids is primarily due to increased kinetic energy. As temperature rises, the kinetic energy of both solute and solvent molecules increases. This increased kinetic energy allows the solvent molecules to more effectively overcome the intermolecular forces holding the solute particles together in the solid state. Think of it like this: the faster-moving solvent molecules have a better chance of colliding with and breaking apart the solute particles, allowing them to dissolve.
Furthermore, the process of dissolving a solid in a liquid is usually endothermic, meaning it absorbs heat. According to Le Chatelier's principle, increasing the temperature shifts the equilibrium towards the endothermic direction, favoring the dissolution process and thus increasing solubility. In simpler terms, adding heat helps "push" the solid into solution.
Let's consider the interactions at a molecular level. When a solid dissolves, the solute-solute interactions (forces between solute particles) and solvent-solvent interactions (forces between solvent particles) are disrupted. New solute-solvent interactions are formed. For many solids, the formation of these new solute-solvent interactions is favored at higher temperatures because the increased kinetic energy helps overcome the initial energy barriers.
Visualizing the Relationship: Solubility Curves
Solubility curves are graphical representations that illustrate the relationship between temperature and solubility for a specific solute in a given solvent. These curves are typically plotted with temperature on the x-axis and solubility (often in g/100g) on the y-axis. The curve itself shows the solubility of the solute at different temperatures. By examining a solubility curve, you can quickly determine the solubility of a substance at a particular temperature, and vice versa. Different substances will have different solubility curves, reflecting their unique interactions with the solvent.
Exceptions to the Rule: Gases and a Few Solids
While the general trend is an increase in solubility with temperature for solids, there are important exceptions. The solubility of gases in liquids generally decreases with increasing temperature. This is because dissolved gases are held in solution by intermolecular forces between the gas molecules and the solvent molecules. As temperature increases, the kinetic energy of the gas molecules increases, allowing them to escape from the solution more easily. Think of a carbonated drink: it goes flat faster when warm because the dissolved carbon dioxide escapes more readily at higher temperatures.
There are also a few unusual solids whose solubility decreases with increasing temperature. These are often exceptions due to complex interactions between the solute and the solvent, and the changes in these interactions with increasing temperature. These cases are less common and typically involve specific chemical systems.
Practical Applications of Temperature-Solubility Relationships
The understanding and application of the temperature-dependence of solubility have far-reaching consequences in numerous fields:
1. Recrystallization in Chemistry
Recrystallization is a purification technique used to isolate a pure solid compound from a mixture. It relies on the principle that the solubility of a solid generally increases with temperature. The impure solid is dissolved in a hot solvent, and as the solution cools, the solubility decreases, causing the purified compound to crystallize out of the solution, leaving behind the impurities in the solution.
2. Pharmaceuticals and Drug Delivery
Solubility is a crucial factor in the development and efficacy of pharmaceutical drugs. Many drugs are solids that need to dissolve in body fluids to be absorbed and exert their therapeutic effects. Understanding the temperature dependence of solubility helps in formulating drugs that have optimal solubility and bioavailability at body temperature. Controlled release formulations often utilize the temperature-sensitive solubility of drugs to regulate the release rate.
3. Environmental Science
The solubility of pollutants in water is affected by temperature. This is particularly important in understanding the fate and transport of pollutants in aquatic environments. For instance, the solubility of many organic pollutants in water decreases with decreasing temperature, potentially leading to increased concentrations of pollutants in cold waters.
4. Geochemistry
Solubility plays a crucial role in geological processes, such as the formation of minerals and rocks. Temperature variations within the Earth’s crust influence the solubility of various minerals, affecting their precipitation, dissolution, and overall distribution. Understanding these temperature-dependent solubilities helps geologists to interpret the formation and evolution of rocks and minerals.
5. Food Science
The solubility of various components in food influences taste, texture, and stability. For instance, the solubility of sugar increases with temperature, making it easier to dissolve in hot beverages. The temperature-dependent solubility of fats and oils affects their behavior during food processing and storage.
Further Considerations: Pressure and Other Factors
While temperature is the dominant factor affecting the solubility of most substances, pressure also plays a role, particularly for gases. Henry's Law states that the solubility of a gas is directly proportional to the partial pressure of the gas above the liquid. Increasing the pressure increases the solubility of gases.
Other factors, such as the presence of other solutes (common ion effect), the pH of the solution, and the polarity of the solvent and solute, also influence solubility. These factors can interact in complex ways, making the prediction of solubility in real-world systems challenging.
Conclusion: A Dynamic Relationship
The relationship between temperature and solubility is a fundamental concept with broad implications across many scientific disciplines and practical applications. While the general rule is that the solubility of most solids increases with temperature, there are exceptions, particularly for gases. Understanding this relationship is crucial for optimizing various processes, from purifying chemicals to formulating drugs to understanding environmental pollution. The detailed study of solubility curves, coupled with an understanding of the underlying principles, allows us to predict and manipulate solubility in numerous ways, enabling advancements in various fields. Future research will continue to refine our understanding of the intricate interplay of factors that govern solubility, leading to further advancements in diverse scientific and technological areas.
Latest Posts
Latest Posts
-
Is Chocolate Chip Cookie Dough Homogeneous Or Heterogeneous
Mar 22, 2025
-
All Tests For Convergence And Divergence
Mar 22, 2025
-
Difference Between Simple And Fractional Distillation
Mar 22, 2025
-
What Is The Polymer For Proteins
Mar 22, 2025
-
Is Copper A Metal Nonmetal Or Metalloid
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about For Most Substances Solubility What As Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
