What Is The Polymer For Proteins
Muz Play
Mar 22, 2025 · 6 min read
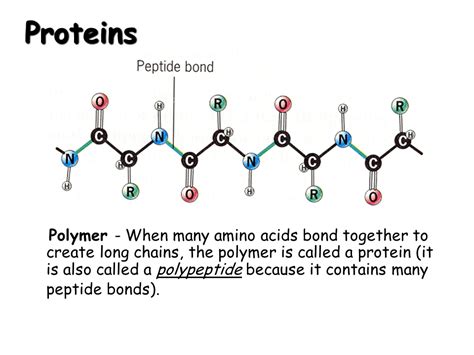
Table of Contents
What is the Polymer for Proteins? Understanding the Building Blocks of Life
Proteins are the workhorses of the cell, carrying out a vast array of functions essential for life. From catalyzing biochemical reactions as enzymes to providing structural support as in collagen, their roles are incredibly diverse. But what exactly are proteins? At their core, proteins are polymers, meaning they are large molecules composed of repeating subunits. This article will delve deep into the nature of proteins as polymers, exploring their building blocks, the types of bonds involved, and the fascinating consequences of their unique structure.
The Monomers of Proteins: Amino Acids
The fundamental building blocks of protein polymers are amino acids. These relatively small organic molecules share a common core structure: a central carbon atom (the alpha carbon) bonded to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom (-H), and a variable side chain, often referred to as the R group. It is this R group that distinguishes one amino acid from another, conferring unique chemical properties and influencing the overall behavior of the protein.
There are 20 standard amino acids commonly found in proteins, each with its own distinct R group. These R groups can be hydrophobic (water-repelling), hydrophilic (water-attracting), positively charged, negatively charged, or polar. This diversity in R group properties is crucial for the diverse functionalities of proteins. For instance:
- Hydrophobic amino acids, like valine and leucine, tend to cluster together in the interior of proteins, away from the aqueous environment of the cell.
- Hydrophilic amino acids, like serine and lysine, often reside on the protein's surface, interacting with the surrounding water molecules.
- Charged amino acids contribute to the protein's overall charge and can participate in electrostatic interactions.
The Diversity of Amino Acid R Groups: A Closer Look
The characteristics of the R group significantly impact the protein's three-dimensional structure and function. Some notable examples include:
- Glycine: The simplest amino acid, with a hydrogen atom as its R group. This makes glycine highly flexible, often found in regions of proteins that require flexibility.
- Proline: Unique in that its R group forms a ring structure with the amino group, restricting its flexibility and often found in turns or bends in the protein structure.
- Cysteine: Contains a sulfhydryl group (-SH) that can form disulfide bonds with other cysteine residues, stabilizing the protein's three-dimensional structure. This is particularly important in proteins secreted outside the cell.
- Tryptophan: Contains a large, aromatic indole ring, often involved in protein-protein interactions and light absorption.
Understanding the properties of these individual amino acids is crucial for comprehending how they assemble into complex protein structures.
Peptide Bonds: Linking Amino Acids Together
Amino acids are linked together to form polypeptide chains through peptide bonds. This is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of another. This reaction releases a water molecule, a process known as a dehydration reaction. The resulting chain of amino acids is called a polypeptide. The sequence of amino acids in a polypeptide chain is determined by the genetic code and is unique to each protein.
Polypeptide Chains: The Backbone and the Side Chains
The polypeptide chain has a distinct backbone structure consisting of repeating -N-C-C- units. The R groups of the amino acids extend outward from this backbone. The sequence of amino acids, their R group properties, and the interactions between them determine the three-dimensional structure of the protein. This structure is crucial for the protein's function.
Levels of Protein Structure: From Primary to Quaternary
The three-dimensional structure of a protein is often described in terms of four levels of organization:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the linear sequence of amino acids in the polypeptide chain. This sequence is dictated by the genetic code and is fundamental to determining the higher levels of structure. Even a single amino acid change can dramatically affect the protein's structure and function.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns within the polypeptide chain stabilized by hydrogen bonds between the backbone atoms. Common secondary structures include:
- Alpha-helices: A coiled structure stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues down the chain.
- Beta-sheets: Extended regions of the polypeptide chain arranged in parallel or antiparallel sheets stabilized by hydrogen bonds between adjacent strands.
- Loops and turns: Regions connecting alpha-helices and beta-sheets, often containing flexible amino acids like glycine and proline.
3. Tertiary Structure: The Overall 3D Arrangement
The tertiary structure describes the overall three-dimensional arrangement of the polypeptide chain, including the spatial relationships between secondary structure elements. This structure is stabilized by a variety of interactions, including:
- Hydrophobic interactions: Nonpolar R groups cluster together in the protein's interior.
- Hydrogen bonds: Occur between polar R groups and other polar molecules.
- Ionic bonds: Form between oppositely charged R groups.
- Disulfide bonds: Covalent bonds formed between cysteine residues.
The tertiary structure is crucial for the protein's function, often creating a specific binding site or active site for interacting with other molecules.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these subunits is referred to as the quaternary structure. The subunits are held together by the same types of interactions that stabilize tertiary structure. Hemoglobin, for example, consists of four subunits that work together to transport oxygen in the blood.
Post-Translational Modifications: Refining the Protein Polymer
Once a protein is synthesized, it can undergo a variety of post-translational modifications. These modifications can alter the protein's structure, function, or stability. Examples include:
- Glycosylation: The addition of carbohydrate groups.
- Phosphorylation: The addition of phosphate groups.
- Proteolytic cleavage: The removal of part of the polypeptide chain.
These modifications add another layer of complexity to the protein polymer, expanding its functional diversity.
The Importance of Protein Structure and Function
The precise three-dimensional structure of a protein is inextricably linked to its function. Even minor alterations in the amino acid sequence or post-translational modifications can significantly impact the protein's ability to carry out its biological role. This is why understanding the polymerization of amino acids into proteins is so critical for comprehending the intricacies of life.
Protein Misfolding and Disease
The delicate balance of interactions that maintain a protein's structure can be disrupted, leading to protein misfolding. Misfolded proteins can lose their function, aggregate to form clumps, and contribute to a range of diseases, including Alzheimer's disease, Parkinson's disease, and cystic fibrosis. Research into protein folding and misfolding is crucial for developing treatments for these debilitating conditions.
Conclusion: The Polymer that Makes Life Possible
Proteins are remarkable biological polymers. The precise polymerization of amino acids, guided by the genetic code and influenced by various interactions and modifications, gives rise to the astounding diversity of protein structure and function. Understanding the principles of protein structure and function is fundamental to a wide range of biological and medical research, from designing new drugs to understanding the pathogenesis of disease. The intricacy of the protein polymer underscores the remarkable complexity and elegance of life itself. Further research continues to reveal new insights into these fascinating molecules and their crucial roles in all living organisms.
Latest Posts
Latest Posts
-
Organic Chemistry Substitution And Elimination Reactions
Mar 23, 2025
-
Arteries Of Lower Limb Flow Chart
Mar 23, 2025
-
What Is The Relation Between Inertia And Mass
Mar 23, 2025
-
1 2 Addition 1 4 Addition
Mar 23, 2025
-
What Is A Row Called In The Periodic Table
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Polymer For Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
