Is Copper A Metal Nonmetal Or Metalloid
Muz Play
Mar 22, 2025 · 6 min read
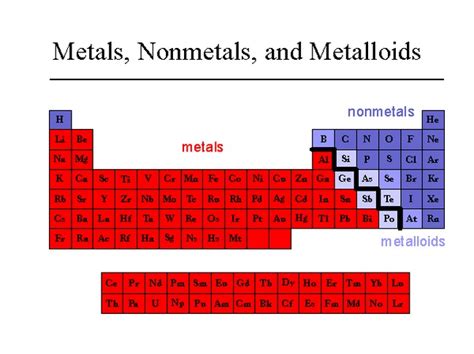
Table of Contents
Is Copper a Metal, Nonmetal, or Metalloid? A Comprehensive Exploration
Copper, a reddish-orange element ubiquitous in our daily lives, is undeniably a metal. This seemingly simple classification belies a fascinating story of its atomic structure, chemical properties, and remarkable versatility. This article will delve deep into the characteristics of copper, explaining why it definitively fits into the metal category and exploring its unique properties that make it so valuable.
Understanding the Periodic Table and Element Classification
Before we definitively classify copper, let's establish a framework for understanding the organization of elements. The periodic table, a cornerstone of chemistry, arranges elements based on their atomic number and recurring chemical properties. Elements are broadly categorized into metals, nonmetals, and metalloids, each exhibiting distinct characteristics.
Metals: A Shared Identity
Metals are typically characterized by:
- Excellent electrical conductivity: They readily conduct electricity due to the ease with which electrons can move through their structure. This property is fundamental to copper's widespread use in electrical wiring.
- High thermal conductivity: Metals efficiently transfer heat. This is why copper is often used in cookware and heat exchangers.
- Malleability and ductility: They can be easily shaped (hammered or rolled into sheets) and drawn into wires without breaking. This property is crucial for copper's use in various manufacturing processes.
- Metallic luster: They have a characteristic shiny appearance. The distinctive reddish-orange sheen of copper is a testament to this property.
- High tensile strength: Many metals possess significant strength and can withstand substantial stress before fracturing.
- Positive oxidation states: They tend to lose electrons readily in chemical reactions.
Nonmetals: A Diverse Group
Nonmetals, in contrast, exhibit properties opposite to those of metals:
- Poor electrical conductivity: They generally do not conduct electricity well.
- Poor thermal conductivity: They are poor conductors of heat.
- Brittle: They tend to be brittle and shatter when subjected to stress.
- Dull appearance: They lack the metallic luster characteristic of metals.
- Negative or non-positive oxidation states: They tend to gain electrons in chemical reactions.
Metalloids: The In-Betweeners
Metalloids, also known as semimetals, represent a transitional group exhibiting properties of both metals and nonmetals. Their behavior can vary significantly depending on the specific conditions. They often possess:
- Semiconductor properties: Their electrical conductivity is intermediate between metals and nonmetals, often increasing with temperature. This property is crucial for their use in electronics.
- Variable physical properties: Their physical properties can fluctuate depending on the specific element and its surrounding conditions.
Copper's Definitive Metallic Characteristics
Now, let's examine copper's characteristics in the context of these classifications. Copper unequivocally displays all the hallmarks of a metal.
Electrical Conductivity: A Cornerstone of Copper's Use
Copper's outstanding electrical conductivity is perhaps its most defining characteristic. Only silver surpasses copper in this property. This exceptional ability to conduct electricity stems from the arrangement of copper's atoms and their loosely held valence electrons. These electrons can move freely throughout the copper lattice, facilitating the flow of electric current. This property underpins copper's extensive use in electrical wiring, power transmission cables, and various electronic components. The efficiency of copper in conducting electricity makes it a cost-effective and reliable material for these applications.
Thermal Conductivity: Efficient Heat Transfer
Similar to its electrical conductivity, copper's excellent thermal conductivity contributes to its widespread applications. Heat readily travels through the copper lattice due to the free movement of electrons. This property makes copper an ideal material for cookware, heat sinks in electronic devices, and heat exchangers in various industrial processes. The ability to efficiently transfer heat is essential for these applications, ensuring optimal performance and preventing overheating.
Malleability and Ductility: Shaping Copper to Our Needs
Copper's malleability (ability to be hammered into thin sheets) and ductility (ability to be drawn into wires) further confirm its metallic nature. These properties allow for the easy shaping and forming of copper into various shapes and forms, making it highly adaptable for diverse applications. From intricate plumbing systems to complex electronic components, the ease with which copper can be manipulated is a key factor in its widespread use.
Metallic Luster: The Characteristic Sheen
Copper possesses the characteristic metallic luster, exhibiting a distinctive reddish-orange sheen. This lustrous appearance is a direct result of the interaction of light with the surface electrons of the metal. This visual characteristic adds to its aesthetic appeal and is used in decorative applications and architectural features.
Oxidation States and Chemical Reactivity
Copper, like other metals, exhibits positive oxidation states in chemical reactions. This signifies its tendency to lose electrons, readily forming compounds with other elements such as oxygen (forming copper oxides) and sulfur (forming copper sulfides). This chemical reactivity, while contributing to its ability to form useful compounds and alloys, also requires consideration in its application, necessitating protective measures against corrosion in certain environments.
Copper Alloys: Expanding the Metal's Versatility
The versatility of copper is further enhanced by its ability to form alloys with other metals. These alloys often exhibit enhanced properties compared to pure copper. Brass (copper and zinc) and bronze (copper and tin) are classic examples, showcasing improved strength, hardness, and corrosion resistance. The formation of these alloys is a direct consequence of copper's metallic bonding and its capacity to interact with other metals to form stable crystalline structures. This ability to form alloys underscores copper's fundamental metallic nature and expands its application across various industries.
Dispelling Misconceptions: Why Copper is Not a Metalloid
Some might mistakenly classify copper as a metalloid due to its role in some semiconductor applications. However, this is a misconception. While copper can be used in certain electronic applications, its primary function in these cases is as a conductor, not as a semiconductor. True metalloids like silicon and germanium exhibit distinct semiconducting properties, where their conductivity is significantly influenced by temperature and the presence of impurities. Copper, on the other hand, remains a highly conductive metal across a wide range of temperatures. Its presence in electronics is predominantly related to its exceptional electrical conductivity and not its semiconducting characteristics.
Conclusion: Copper's Undeniable Metallic Identity
In conclusion, the evidence overwhelmingly supports the classification of copper as a metal. Its exceptional electrical and thermal conductivity, malleability, ductility, metallic luster, and positive oxidation states all align perfectly with the characteristic properties of metals. While its use in some electronic applications might cause confusion, its fundamental behavior consistently affirms its position as a quintessential metal. The diverse applications of copper, from electrical wiring to cookware to alloys, are a testament to its remarkable versatility and the inherent strengths of its metallic nature. Understanding the clear distinction between metals, nonmetals, and metalloids allows for a deeper appreciation of copper's unique properties and its indispensable role in various aspects of our modern world.
Latest Posts
Latest Posts
-
5 4 Practice Analyzing Graphs Of Polynomial Functions
Mar 23, 2025
-
Why Do Ionic Compounds Have High Melting Point
Mar 23, 2025
-
Are Shield Volcanoes Mafic Or Felsic
Mar 23, 2025
-
Does Sulfur Follow The Octet Rule
Mar 23, 2025
-
Temperature And Kinetic Energy Have A Relationship
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Copper A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
