Does Sulfur Follow The Octet Rule
Muz Play
Mar 23, 2025 · 6 min read
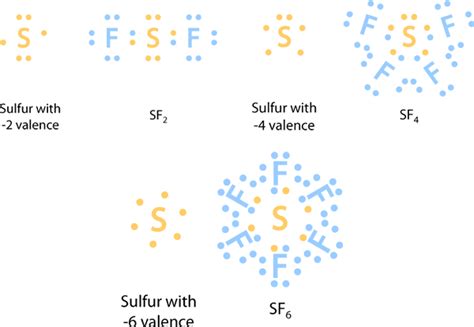
Table of Contents
Does Sulfur Follow the Octet Rule? Exploring the Exceptions to the Rule
The octet rule, a cornerstone of basic chemistry, states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their valence shell, achieving a stable electron configuration resembling that of a noble gas. While this rule serves as a useful guideline for understanding bonding in many compounds, it's crucial to recognize that it's not a hard and fast law. Sulfur, a fascinating element with diverse chemical behavior, provides a compelling example of an element that frequently deviates from the octet rule. This article delves into the intricacies of sulfur's bonding, exploring when it adheres to the octet rule and, more importantly, when and why it doesn't.
Sulfur's Electron Configuration and Bonding Capacity
Sulfur (S), with an atomic number of 16, possesses the electron configuration [Ne]3s²3p⁴. This means it has six electrons in its valence shell. To achieve a stable octet, sulfur needs to gain two electrons. This explains its common -2 oxidation state in compounds like hydrogen sulfide (H₂S) and many metal sulfides. In these cases, sulfur forms two covalent bonds, completing its octet.
Examples of Sulfur Following the Octet Rule
-
Hydrogen Sulfide (H₂S): Sulfur forms two single covalent bonds with two hydrogen atoms, sharing two electrons with each hydrogen and completing its octet. The Lewis structure clearly demonstrates this adherence to the rule.
-
Sulfur Dioxide (SO₂): While the Lewis structure of SO₂ initially appears to violate the octet rule (with sulfur having only six valence electrons), resonance structures can be drawn that show sulfur sharing electrons with both oxygen atoms, resulting in a completed octet across resonance forms.
-
Many Metal Sulfides (e.g., FeS, ZnS): In these ionic compounds, sulfur gains two electrons from the metal cation, achieving a stable octet and a -2 oxidation state.
When Sulfur Defies the Octet Rule: Expanding the Valence Shell
Sulfur, unlike some other elements, exhibits a remarkable ability to expand its valence shell. This means it can accommodate more than eight electrons in its outermost shell, leading to hypervalent molecules. This ability is a consequence of its access to empty 3d orbitals. These orbitals, while higher in energy than the 3s and 3p orbitals, can participate in bonding, allowing sulfur to form more than four covalent bonds.
Understanding the Role of d-Orbitals
The involvement of d-orbitals in bonding is a key factor enabling sulfur's hypervalency. While the energy difference between the 3p and 3d orbitals isn't negligible, it's small enough that under certain circumstances, the energy gain from forming additional bonds outweighs the energy cost of promoting electrons into the 3d orbitals. This explains why sulfur can form compounds with more than eight electrons in its valence shell.
Examples of Hypervalent Sulfur Compounds
-
Sulfur Hexafluoride (SF₆): This is a classic example of a hypervalent molecule. Sulfur forms six covalent bonds with six fluorine atoms, resulting in a total of 12 valence electrons around the sulfur atom – a clear violation of the octet rule. The expanded octet is made possible by the participation of its 3d orbitals.
-
Sulfuric Acid (H₂SO₄): In sulfuric acid, sulfur is bonded to four oxygen atoms, again exceeding the octet. Two of these bonds are double bonds, while two are single bonds. The involvement of d-orbitals is crucial in explaining the formation of these multiple bonds.
-
Thionyl Chloride (SOCl₂): This compound showcases sulfur's flexibility in bonding. It features a double bond to oxygen and two single bonds to chlorine atoms, leading to an expanded octet around the sulfur atom.
Factors Influencing Octet Rule Adherence in Sulfur Compounds
Several factors influence whether sulfur will adhere to or deviate from the octet rule:
-
Electronegativity of the bonded atom: When bonding with highly electronegative atoms like fluorine, chlorine, or oxygen, sulfur is more likely to expand its octet. These electronegative atoms effectively withdraw electron density from sulfur, making it energetically favorable for sulfur to utilize its d-orbitals.
-
Steric factors: The size and arrangement of atoms bonded to sulfur can also affect its bonding behavior. Steric hindrance can influence the formation of hypervalent compounds.
-
Bond energies: The overall stability of the molecule also plays a critical role. The formation of additional bonds, even if it results in an expanded octet, can be energetically favorable if the resulting molecule is significantly more stable than one that adheres strictly to the octet rule.
The Limitations of the Octet Rule and its Importance
It's essential to remember that the octet rule is a useful simplification, not an absolute law. While it accurately predicts the bonding in many simple compounds, it fails to account for the diverse bonding behavior of elements like sulfur, phosphorus, and other elements in the third period and beyond. These elements can readily expand their valence shells due to the availability of d-orbitals.
Understanding the limitations of the octet rule is vital for a comprehensive grasp of chemical bonding. While it's a useful starting point, it should not be considered a rigid constraint. The ability of sulfur to expand its octet explains its remarkable versatility in forming a wide range of compounds with diverse structures and properties.
Advanced Concepts and Further Exploration
The bonding in hypervalent molecules like those formed by sulfur is a complex topic, and various theoretical models attempt to explain it. These include:
-
3-center 4-electron bonds: These are delocalized bonds that involve three atoms and four electrons. They're often invoked to explain the bonding in some hypervalent molecules, although their precise nature is still a subject of ongoing discussion.
-
Molecular orbital theory: This more sophisticated approach to bonding provides a more detailed picture of the electron distribution in hypervalent molecules. It accounts for the interaction of atomic orbitals to form molecular orbitals that span the entire molecule.
By exploring the intricacies of sulfur's bonding, we gain a deeper understanding of the nuances of chemical bonding and the limitations of simplified rules like the octet rule. The ability of sulfur to both adhere to and deviate from this rule highlights its versatility and the richness of chemical behavior in the world around us. Further investigation into these advanced concepts provides a stronger foundation for understanding the complexity and elegance of chemical bonding. The field continues to evolve with new research and theoretical models refining our comprehension of these fascinating chemical phenomena. This ongoing exploration underscores the importance of critical thinking and the willingness to question even seemingly fundamental principles in the pursuit of scientific understanding.
Latest Posts
Latest Posts
-
Conservation Of Power In A Circuit
Mar 24, 2025
-
What Is The Relationship Between Absorbance And Concentration
Mar 24, 2025
-
How To Multiply Vector Wise Equations
Mar 24, 2025
-
Introduction To Curriculum For Early Childhood Education
Mar 24, 2025
-
What Is Meant By The Statement Enzymes Are Biological Catalysts
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Does Sulfur Follow The Octet Rule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
