What Is Meant By The Statement Enzymes Are Biological Catalysts
Muz Play
Mar 24, 2025 · 6 min read
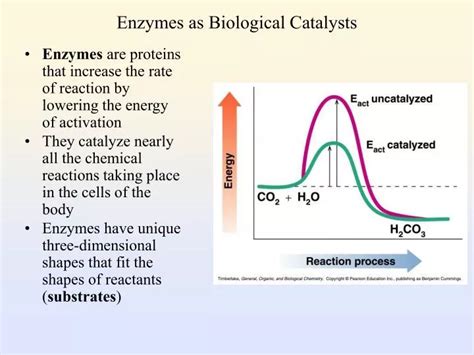
Table of Contents
What is Meant by the Statement: Enzymes are Biological Catalysts?
Enzymes are remarkable molecules, the workhorses of life. Their crucial role is to speed up, or catalyze, biological reactions within living organisms. Understanding the statement "enzymes are biological catalysts" requires delving into both the nature of enzymes themselves and the fundamental principles of catalysis. This article will explore this statement comprehensively, covering enzyme structure, function, mechanisms, and their importance in various biological processes.
Understanding Catalysts
Before diving into the specifics of enzymes, let's establish a clear understanding of what a catalyst is. A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed in the process. This means that after the reaction is complete, the catalyst remains unchanged and can be used again to catalyze the same reaction repeatedly. Catalysts achieve this acceleration by lowering the activation energy of the reaction.
Activation Energy: The Energy Barrier
Chemical reactions, even spontaneous ones (those that release energy), require a certain amount of energy to get started. This initial energy input is called the activation energy (Ea). Think of it like pushing a boulder over a hill – you need to exert energy to get it moving over the initial hump. Once it's over the hump, it rolls down the other side, releasing energy. The activation energy represents the energy barrier that must be overcome for reactants to transform into products.
How Catalysts Lower Activation Energy
Catalysts work by providing an alternative reaction pathway with a lower activation energy. Instead of the reactants needing to overcome the high energy barrier of the uncatalyzed reaction, the catalyst provides a "shortcut," a lower-energy route to the products. This allows the reaction to proceed much faster at a given temperature. Importantly, the catalyst does not change the overall energy difference between reactants and products (the ΔG, or Gibbs Free Energy change); it only affects the rate of the reaction.
Enzymes: Biological Catalysts
Now, let's bring the concept of catalysts into the biological realm. Enzymes are biological catalysts, meaning they are proteins (mostly) that speed up biochemical reactions within cells. They are highly specific, meaning they typically catalyze only one type of reaction or a very limited set of similar reactions. This specificity is crucial for maintaining the precise control and regulation required for life's complex processes.
The Structure of Enzymes: Form Follows Function
The remarkable catalytic activity of enzymes stems from their highly specific three-dimensional structures. Enzymes are typically globular proteins, folded into intricate shapes with specific regions called active sites. The active site is a pocket or cleft on the enzyme's surface where the substrate (the molecule being acted upon) binds. The precise arrangement of amino acid residues within the active site is crucial for substrate recognition and catalysis.
The three-dimensional structure of an enzyme is not rigid; it is flexible and can undergo conformational changes upon substrate binding. This induced fit model explains how the enzyme adjusts its shape to optimally interact with the substrate, creating a highly specific and efficient interaction.
The Enzyme-Substrate Complex
When a substrate binds to the enzyme's active site, it forms an enzyme-substrate (ES) complex. This complex represents a transient intermediate state where the enzyme and substrate are in close proximity, allowing the catalytic process to occur. The enzyme facilitates the conversion of the substrate into the product, which is then released from the active site, leaving the enzyme free to catalyze another reaction.
Mechanisms of Enzyme Catalysis
Enzymes employ a variety of mechanisms to accelerate reactions. These include:
-
Proximity and Orientation: Enzymes bring substrates together in the correct orientation for reaction, increasing the probability of successful collisions. This is particularly important for bimolecular reactions (reactions involving two molecules).
-
Acid-Base Catalysis: Amino acid residues in the active site act as acids or bases, donating or accepting protons to facilitate the reaction.
-
Covalent Catalysis: The enzyme forms a temporary covalent bond with the substrate, creating a reaction intermediate that is more readily converted to the product.
-
Metal Ion Catalysis: Metal ions, often bound to the active site, can participate in various catalytic roles, such as facilitating electron transfer or stabilizing reaction intermediates.
-
Strain and Distortion: Enzymes can bind substrates in a strained or distorted conformation, making them more reactive.
Factors Affecting Enzyme Activity
The rate of enzyme-catalyzed reactions is influenced by several factors:
-
Substrate Concentration: At low substrate concentrations, the reaction rate increases linearly with increasing substrate concentration. However, at high substrate concentrations, the rate plateaus as the enzyme becomes saturated – all active sites are occupied.
-
Enzyme Concentration: Increasing the enzyme concentration increases the reaction rate, as there are more active sites available to bind substrates.
-
Temperature: Enzymes have optimal temperature ranges. At low temperatures, the reaction rate is slow due to low kinetic energy. At high temperatures, enzymes can denature (lose their three-dimensional structure), resulting in loss of activity.
-
pH: Each enzyme has an optimal pH range. Deviations from the optimal pH can alter the enzyme's structure and activity.
-
Inhibitors: Inhibitors are molecules that bind to enzymes and reduce their activity. Competitive inhibitors compete with the substrate for binding to the active site, while non-competitive inhibitors bind to a different site on the enzyme, altering its conformation and reducing its activity.
-
Activators: Conversely, activators are molecules that increase enzyme activity, often by binding to allosteric sites and inducing conformational changes that enhance substrate binding or catalysis.
The Importance of Enzymes in Biological Processes
Enzymes are essential for virtually every aspect of life. Their catalytic activity is critical for:
-
Metabolism: Enzymes catalyze the countless reactions involved in energy production, nutrient breakdown, and biosynthesis of essential molecules. This includes glycolysis, the citric acid cycle, and oxidative phosphorylation.
-
DNA Replication and Repair: Enzymes such as DNA polymerase and ligase are crucial for accurate DNA replication and repair, ensuring genetic stability.
-
Protein Synthesis: Ribosomes, RNA polymerases, and aminoacyl-tRNA synthetases are essential enzymes in the complex process of protein synthesis, translating genetic information into functional proteins.
-
Signal Transduction: Enzymes play a key role in signal transduction pathways, relaying information from the environment to the inside of the cell. Kinases and phosphatases are important examples of enzymes involved in these pathways.
-
Digestion: Enzymes such as amylase, protease, and lipase are critical for breaking down food molecules into smaller, absorbable units.
-
Immune Response: Enzymes play a vital role in the immune response, facilitating processes like antibody production and pathogen destruction.
Enzyme Engineering and Applications
The understanding of enzyme structure and function has led to significant advancements in enzyme engineering. This field focuses on modifying enzymes to enhance their properties, such as catalytic efficiency, stability, or specificity. Engineered enzymes find wide applications in various industries, including:
-
Biotechnology: Enzymes are used in numerous biotechnological processes, such as the production of biofuels, pharmaceuticals, and industrial chemicals.
-
Medicine: Enzymes are used as therapeutic agents, diagnostic tools, and in biosensors.
-
Food Industry: Enzymes are used to improve food processing, such as in baking, brewing, and cheese making.
-
Environmental Remediation: Enzymes are employed in bioremediation processes to break down pollutants.
Conclusion: The Ubiquity and Importance of Enzymatic Catalysis
The statement "enzymes are biological catalysts" encapsulates a fundamental truth about the nature of life. These remarkable molecules are essential for almost all biological processes, driving the incredible complexity and efficiency of living systems. Their remarkable specificity, catalytic power, and regulatory roles are testament to the elegance and precision of biological chemistry. Understanding their mechanisms and properties is crucial for advancing our knowledge of biology and its applications in various fields. From understanding disease mechanisms to developing new technologies, the study of enzymes remains a vibrant and crucial area of scientific research.
Latest Posts
Latest Posts
-
How To Multiply And Divide Rational Expressions
Mar 29, 2025
-
Difference Between Lewis Acid And Bronsted Acid
Mar 29, 2025
-
Which Of The Following Orbitals Is The Last To Fill
Mar 29, 2025
-
What Is A Fluid Connective Tissue
Mar 29, 2025
-
What Is The Van Der Waals Equation
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is Meant By The Statement Enzymes Are Biological Catalysts . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
