Difference Between Lewis Acid And Bronsted Acid
Muz Play
Mar 29, 2025 · 6 min read
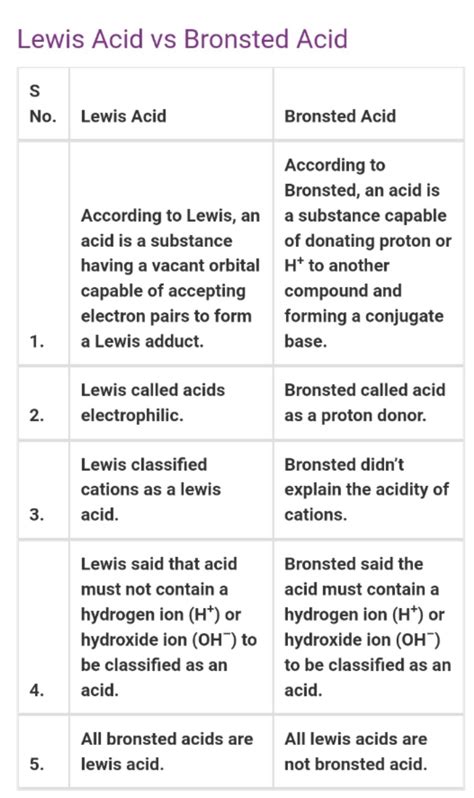
Table of Contents
Delving Deep into the Differences: Lewis Acids vs. Brønsted-Lowry Acids
Understanding the nuances of acid-base chemistry is crucial for anyone studying chemistry, from high school students to seasoned researchers. While the Brønsted-Lowry definition of acids and bases is widely taught, the Lewis definition provides a broader and more encompassing perspective. This article dives deep into the differences between Lewis acids and Brønsted-Lowry acids, exploring their definitions, examples, and applications.
The Brønsted-Lowry Definition: Proton Transfer
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines acids and bases based on proton transfer.
-
Brønsted-Lowry Acid: A Brønsted-Lowry acid is a substance that donates a proton (H⁺) to another substance. This donation process often involves the breaking of an O-H or N-H bond.
-
Brønsted-Lowry Base: A Brønsted-Lowry base is a substance that accepts a proton (H⁺) from another substance. This acceptance usually involves the formation of an O-H or N-H bond.
Key Characteristics of Brønsted-Lowry Acid-Base Reactions:
- Proton Transfer: The defining characteristic is the transfer of a proton from the acid to the base.
- Conjugate Acid-Base Pairs: The acid loses a proton to form its conjugate base, and the base gains a proton to form its conjugate acid. These pairs differ by a single proton.
- Water as a Solvent: Many Brønsted-Lowry acid-base reactions occur in aqueous solutions, with water acting as either an acid or a base depending on the other reactant.
Examples of Brønsted-Lowry Acids:
- Hydrochloric acid (HCl): HCl donates a proton to water, forming H₃O⁺ (hydronium ion) and Cl⁻.
- Sulfuric acid (H₂SO₄): A strong diprotic acid that can donate two protons.
- Acetic acid (CH₃COOH): A weak monoprotic acid commonly found in vinegar.
- Ammonium ion (NH₄⁺): Donates a proton to form ammonia (NH₃).
Examples of Brønsted-Lowry Bases:
- Ammonia (NH₃): Accepts a proton from water, forming NH₄⁺ and OH⁻ (hydroxide ion).
- Sodium hydroxide (NaOH): A strong base that dissociates in water to produce OH⁻ ions.
- Water (H₂O): Can act as both an acid and a base (amphoteric).
The Lewis Definition: Electron Pair Acceptance
The Lewis definition, introduced by Gilbert N. Lewis in 1923, provides a broader perspective on acid-base reactions by focusing on electron pair interactions.
-
Lewis Acid: A Lewis acid is a substance that accepts a pair of electrons to form a covalent bond. They are often electron-deficient species with an empty orbital that can accommodate a lone pair of electrons.
-
Lewis Base: A Lewis base is a substance that donates a pair of electrons to form a covalent bond. They usually possess a lone pair of electrons that can be shared.
Key Characteristics of Lewis Acid-Base Reactions:
- Electron Pair Donation and Acceptance: The central feature is the donation of an electron pair from the base to the acid, forming a coordinate covalent bond (also known as a dative bond).
- No Proton Transfer Required: Unlike Brønsted-Lowry reactions, Lewis acid-base reactions don't necessarily involve proton transfer. This makes the Lewis definition more inclusive.
- Variety of Reactants: The Lewis definition encompasses a wider range of reactants, including those that don't contain protons.
Examples of Lewis Acids:
- Boron trifluoride (BF₃): Has an empty p-orbital and readily accepts an electron pair.
- Aluminum chloride (AlCl₃): Similar to BF₃, it acts as a Lewis acid due to its electron deficiency.
- Iron(III) ion (Fe³⁺): A metal cation with a high positive charge that can attract electron pairs.
- Carbon dioxide (CO₂): The carbon atom can accept electron pairs from bases.
- Transition metal ions: Many transition metal ions act as Lewis acids, forming complexes with ligands (Lewis bases).
Examples of Lewis Bases:
- Ammonia (NH₃): Possesses a lone pair of electrons on the nitrogen atom.
- Water (H₂O): Has two lone pairs of electrons on the oxygen atom.
- Chloride ion (Cl⁻): Has lone pairs of electrons.
- Carbon monoxide (CO): The carbon atom possesses a lone pair of electrons.
Comparing Brønsted-Lowry and Lewis Acids
The key difference lies in their focus:
| Feature | Brønsted-Lowry Acid | Lewis Acid |
|---|---|---|
| Definition | Proton (H⁺) donor | Electron pair acceptor |
| Mechanism | Proton transfer | Electron pair donation and acceptance |
| Proton Transfer | Always involves proton transfer | Does not necessarily involve proton transfer |
| Scope | More limited scope | Broader scope; includes many substances not classified as acids by Brønsted-Lowry |
Overlapping Cases:
It's crucial to understand that all Brønsted-Lowry acids are also Lewis acids. This is because the proton (H⁺) accepts an electron pair from the base during the proton transfer. However, many Lewis acids are not Brønsted-Lowry acids because they don't donate protons.
Applications and Significance
Understanding the differences between Lewis and Brønsted-Lowry acids is crucial in various fields:
- Organic Chemistry: Lewis acids are frequently used as catalysts in many organic reactions, such as Friedel-Crafts alkylation and acylation. Their ability to accept electron pairs allows them to activate reactants and facilitate the formation of new bonds.
- Inorganic Chemistry: The formation of coordination complexes is a quintessential example of Lewis acid-base chemistry. Transition metal ions (Lewis acids) form bonds with ligands (Lewis bases) to create stable complexes with diverse structures and properties. This is fundamental to many applications, including catalysis and materials science.
- Biochemistry: Many biological processes involve Lewis acid-base interactions. Enzyme-substrate interactions often involve Lewis acid-base interactions, where the enzyme acts as a Lewis acid and the substrate as a Lewis base. Metal ions play crucial roles in many metalloenzymes, acting as Lewis acids in catalysis.
- Analytical Chemistry: Acid-base titrations are a fundamental technique used to determine the concentration of unknown acids or bases. While Brønsted-Lowry titrations are commonly employed, the Lewis definition allows for a broader understanding of the interactions involved.
Beyond the Basics: Exploring Nuances
While the definitions presented offer a clear understanding of the core differences, there are subtle complexities worth exploring:
- Hardness and Softness: The concept of "hard" and "soft" acids and bases adds another layer of understanding. Hard acids prefer to interact with hard bases (e.g., oxygen and nitrogen), while soft acids prefer soft bases (e.g., sulfur and phosphorus). This is related to the polarizability of the acid and base.
- Solvent Effects: The solvent can significantly influence the behavior of acids and bases. The strength of an acid or base can vary depending on the solvent used.
- Quantitative Measures: The strength of Brønsted-Lowry acids is often expressed using pKa values, indicating the tendency to donate a proton. While there isn't a universally accepted equivalent for Lewis acids, relative strengths can be assessed based on their ability to form complexes with different bases.
Conclusion: A Unified View of Acidity
The Brønsted-Lowry and Lewis definitions offer complementary perspectives on acid-base chemistry. While the Brønsted-Lowry definition provides a simpler framework for many common acid-base reactions involving proton transfer, the Lewis definition offers a broader and more inclusive framework that encompasses a much wider range of chemical interactions. Understanding both definitions is essential for a thorough understanding of acid-base chemistry and its vast applications across different scientific disciplines. By appreciating the similarities and differences, we obtain a more complete and nuanced comprehension of this fundamental concept in chemistry. The combined understanding allows us to predict and explain a vast array of chemical phenomena and build a stronger foundation for advanced chemical studies.
Latest Posts
Latest Posts
-
Cracking The Code Of Life Answer Key
Mar 31, 2025
-
Delta H Is Negative Exothermic Or Endothermic
Mar 31, 2025
-
Raising And Lowering Operators Angular Momentum
Mar 31, 2025
-
Unauthrized Data Rollbak Or Undo Cia Triad
Mar 31, 2025
-
Titration Curve Of Naoh And Hcl
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Lewis Acid And Bronsted Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
