Why Do Ionic Compounds Have High Melting Point
Muz Play
Mar 23, 2025 · 6 min read
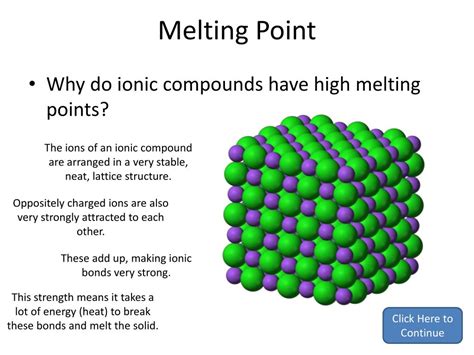
Table of Contents
Why Do Ionic Compounds Have High Melting Points? A Deep Dive into Electrostatic Forces
Ionic compounds, characterized by the strong electrostatic attraction between oppositely charged ions, exhibit significantly higher melting points compared to covalent compounds. Understanding this requires a thorough examination of the forces holding these compounds together and the energy required to overcome them. This article delves into the intricacies of ionic bonding, exploring the factors contributing to the high melting points observed in these materials. We'll explore the role of lattice energy, Coulomb's law, and the influence of factors like ion charge and size on the strength of the ionic bond and consequently, the melting point.
The Foundation: Ionic Bonding and Lattice Structure
Ionic bonding arises from the electrostatic attraction between a cation (positively charged ion) and an anion (negatively charged ion). This transfer of electrons, typically from a metal to a nonmetal, creates ions with complete outermost electron shells, achieving a stable electronic configuration. The resulting electrostatic force is exceptionally strong, leading to the formation of a highly ordered three-dimensional structure known as a crystal lattice.
The Crystal Lattice: A Highly Ordered Arrangement
Imagine a vast, intricate network where positive and negative ions are arranged in a precise, repeating pattern. This is the essence of the ionic crystal lattice. The arrangement minimizes the repulsive forces between ions of like charge while maximizing attractive forces between ions of opposite charge. This highly ordered structure is responsible for many of the characteristic properties of ionic compounds, including their high melting points.
The Key Player: Lattice Energy
Lattice energy is the energy released when gaseous ions combine to form one mole of a solid ionic compound. Conversely, it represents the energy required to completely separate one mole of an ionic solid into its constituent gaseous ions. The magnitude of lattice energy directly correlates with the melting point of an ionic compound. A higher lattice energy implies stronger ionic bonds, requiring more energy to break these bonds and thus resulting in a higher melting point.
Coulomb's Law: Quantifying the Electrostatic Attraction
The strength of the electrostatic attraction between ions is governed by Coulomb's Law:
F = k * (q1 * q2) / r²
Where:
- F represents the force of attraction.
- k is Coulomb's constant.
- q1 and q2 are the magnitudes of the charges of the ions.
- r is the distance between the centers of the ions.
This equation reveals several crucial factors influencing the strength of ionic bonds and, consequently, the melting point:
-
Charge of the Ions: Higher ionic charges lead to stronger electrostatic attractions. A compound with ions carrying a 2+ charge and a 2− charge will experience a significantly stronger attractive force than a compound with 1+ and 1− ions.
-
Distance Between Ions (Ionic Radii): Smaller ions, positioned closer together, experience a stronger attractive force than larger ions separated by greater distances. The inverse square relationship in Coulomb's Law highlights the importance of ionic size. As the distance increases, the force of attraction decreases dramatically.
Factors Affecting Melting Points: A Detailed Exploration
Let's delve into how the charge and size of ions directly impact the melting points of ionic compounds.
The Impact of Ionic Charge
Consider the following examples:
-
NaCl (Sodium Chloride): Na+ and Cl− ions have single charges, resulting in a relatively moderate lattice energy and melting point (801°C).
-
MgO (Magnesium Oxide): Mg2+ and O2− ions possess double charges, leading to a significantly higher lattice energy and a much higher melting point (2852°C).
The increased charge magnitude in MgO results in a substantially stronger electrostatic attraction, demanding far more energy to overcome the ionic bonds and thus leading to a drastically higher melting point.
The Role of Ionic Radius
Now, let's analyze the influence of ionic size:
-
LiF (Lithium Fluoride): Li+ and F− ions are relatively small, leading to a short interionic distance and a strong attractive force, resulting in a high melting point (845°C).
-
KCl (Potassium Chloride): K+ and Cl− ions are larger than Li+ and F−, resulting in a longer interionic distance and a weaker attractive force, leading to a lower melting point (770°C).
Even with similar charges, the increased ionic radii in KCl weaken the electrostatic attraction compared to LiF, requiring less energy to disrupt the lattice and consequently resulting in a lower melting point.
Beyond Charge and Size: Other Contributing Factors
While charge and size are dominant factors, other subtle influences can affect melting points:
-
Polarizability of Ions: Larger ions with more diffuse electron clouds are more polarizable. This means their electron distribution can be distorted by the electric field of neighboring ions, leading to additional attractive forces and slightly higher melting points.
-
Lattice Structure: Different crystal lattice structures (e.g., cubic close-packed, face-centered cubic) have different arrangements of ions, which can slightly alter the overall lattice energy and melting point.
-
Covalent Character: In some ionic compounds, there can be a degree of covalent character to the bonding, which can subtly affect the melting point. This is particularly true when the difference in electronegativity between the cation and anion is not extremely large.
Comparing Ionic and Covalent Compounds: A Clear Distinction
The high melting points of ionic compounds stand in stark contrast to the relatively low melting points of covalent compounds. This difference arises from the fundamental nature of the bonding:
-
Ionic Compounds: The strong electrostatic forces in the ionic lattice require a significant amount of energy to overcome, resulting in high melting points.
-
Covalent Compounds: Covalent bonds involve the sharing of electrons between atoms. These bonds are generally weaker than ionic bonds, requiring less energy to break, leading to lower melting points.
The energy required to break the numerous strong ionic bonds in a crystal lattice is substantial, clearly explaining the elevated melting points observed in ionic compounds.
Practical Applications and Significance
The high melting points of ionic compounds are exploited in various applications:
-
High-Temperature Materials: Ionic compounds like MgO (magnesia) and Al2O3 (alumina) are used as refractory materials in furnaces and high-temperature applications due to their exceptional thermal stability.
-
Electrolytes: Many ionic compounds readily dissolve in polar solvents, dissociating into ions and conducting electricity. This property is crucial in batteries and other electrochemical devices.
-
Ceramics: Ionic compounds form the basis of many ceramic materials, which are known for their hardness, strength, and resistance to wear and corrosion.
Understanding the high melting points of ionic compounds is essential in material science, chemistry, and various engineering disciplines. The ability to manipulate the properties of ionic materials through careful control of ion charge, size, and lattice structure allows for the design and development of new materials with specific functionalities and applications.
Conclusion: A Powerful Bond with High Thermal Stability
The high melting points of ionic compounds are a direct consequence of the strong electrostatic forces holding the ions together within the crystal lattice. Coulomb's Law elegantly explains the relationship between ionic charge, interionic distance, and the strength of the ionic bond. The magnitude of the lattice energy, a direct measure of this bond strength, dictates the amount of energy required to overcome these attractions and transition from the solid to the liquid phase. By understanding these fundamental principles, we gain insights into the remarkable properties of ionic compounds and their wide-ranging applications. Further research continues to explore the nuances of ionic bonding, promising the development of advanced materials with even more impressive thermal stability and other desirable characteristics.
Latest Posts
Latest Posts
-
Is A Molecule Smaller Than An Atom
Mar 24, 2025
-
How To Calculate Total Magnification On A Microscope
Mar 24, 2025
-
How To Find The Frequency Of Oscillation
Mar 24, 2025
-
A Perfectly Elastic Supply Curve Is
Mar 24, 2025
-
Bones Of The Pectoral Girdle And Upper Limb
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Why Do Ionic Compounds Have High Melting Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
