Is A Molecule Smaller Than An Atom
Muz Play
Mar 24, 2025 · 5 min read
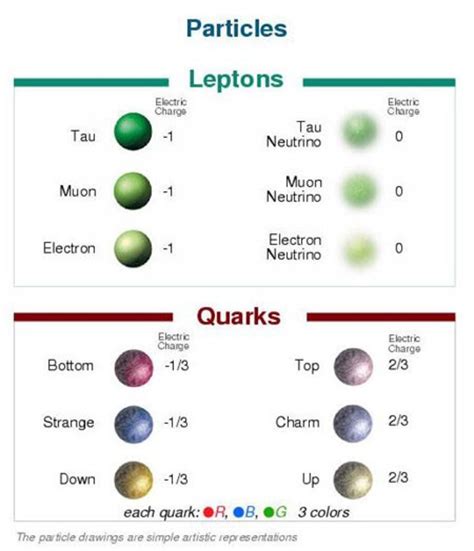
Table of Contents
Is a Molecule Smaller Than an Atom? Understanding the Building Blocks of Matter
The question, "Is a molecule smaller than an atom?" is a common one, often arising from a basic misunderstanding of the fundamental building blocks of matter. The simple answer is no, a molecule is generally not smaller than an atom. In fact, it's quite the opposite. Atoms are the fundamental units of matter, and molecules are made up of multiple atoms bonded together. Let's delve deeper into the fascinating world of atoms and molecules to clarify this misconception and explore the relationships between them.
Atoms: The Indivisible Units?
The word "atom" comes from the Greek word "atomos," meaning "indivisible." For a long time, atoms were indeed considered the smallest units of matter, the ultimate building blocks. However, modern science has revealed a much more complex internal structure. Atoms are comprised of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus.
- Neutrons: Neutrally charged particles also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The number of protons defines the element. For example, an atom with one proton is hydrogen, two protons is helium, and so on. The number of neutrons can vary within an element, leading to isotopes, which are atoms of the same element with differing neutron counts. Electrons play a crucial role in chemical bonding, which is the force that holds atoms together to form molecules.
Molecules: The Union of Atoms
A molecule is formed when two or more atoms chemically bond together. This bonding involves the sharing or transfer of electrons between atoms. There are several types of chemical bonds, including:
-
Covalent Bonds: These bonds are formed when atoms share electrons. This sharing creates a strong attraction between the atoms, holding them together. Many organic molecules, such as those found in living organisms, are held together by covalent bonds. Examples include water (H₂O), carbon dioxide (CO₂), and glucose (C₆H₁₂O₆).
-
Ionic Bonds: These bonds are formed when one atom transfers one or more electrons to another atom. This transfer creates ions: positively charged cations and negatively charged anions. The electrostatic attraction between these oppositely charged ions forms the ionic bond. Table salt (NaCl), or sodium chloride, is a classic example of an ionic compound.
-
Metallic Bonds: These bonds occur between metal atoms. Electrons are delocalized, meaning they are not associated with a specific atom but rather move freely throughout the metal structure. This explains the characteristic properties of metals, such as conductivity and malleability.
The size of a molecule depends on the number and type of atoms it contains, as well as the type of bonds holding them together. Generally, a molecule is significantly larger than a single atom because it comprises multiple atoms. The bonds between the atoms contribute to the overall size and shape of the molecule.
Comparing Sizes: Atoms vs. Molecules
To illustrate the size difference, consider a simple water molecule (H₂O). A single water molecule is comprised of two hydrogen atoms and one oxygen atom. The oxygen atom is significantly larger than the hydrogen atoms. The overall size of the water molecule is, therefore, larger than either a single hydrogen atom or a single oxygen atom.
Similarly, complex biological molecules like proteins and DNA are composed of thousands or even millions of atoms. These macromolecules are incredibly large compared to individual atoms.
The Importance of Understanding Atomic and Molecular Structure
Understanding the difference between atoms and molecules is fundamental to various fields of science, including:
-
Chemistry: The study of matter and its properties is fundamentally based on the understanding of atomic and molecular structures and their interactions.
-
Biology: Living organisms are built from complex molecules, such as proteins, carbohydrates, and nucleic acids. Understanding molecular structures is crucial to understanding biological processes.
-
Physics: Atomic and molecular physics explores the behavior of atoms and molecules, including their energy levels and interactions with light and other forms of radiation.
-
Materials Science: The properties of materials are largely determined by their atomic and molecular structure. Understanding this structure is crucial for designing and creating new materials with specific properties.
Addressing Common Misconceptions
The misconception that molecules are smaller than atoms often stems from a lack of visual representation and a simplified understanding of atomic structure. We rarely see atoms and molecules directly; their visualization usually involves models and representations. These models may sometimes simplify the complexity of these structures, potentially leading to confusion.
It's also important to remember that atoms aren't solid spheres as they are often depicted in simple diagrams. They have a complex structure with a relatively small, dense nucleus surrounded by a vast, mostly empty space where electrons are located. This space is where the bonding interactions take place, forming molecules and determining their size and shape.
Beyond Atoms and Molecules: Subatomic Particles and Beyond
While atoms are the fundamental building blocks of chemical reactions and the formation of molecules, the exploration of matter doesn't stop there. Delving deeper into the subatomic world reveals even smaller particles like quarks and leptons, which constitute protons, neutrons, and electrons. Understanding these fundamental particles requires venturing into the realm of quantum mechanics, a complex and fascinating field of physics.
The structure of matter extends beyond atoms and molecules to encompass various levels of organization, including:
-
Compounds: These are substances formed by the chemical combination of two or more different elements in fixed proportions. Compounds are often, but not always, molecules. For example, table salt (NaCl) is a compound but is not a molecule in the traditional sense, as it exists as a crystalline lattice of ions.
-
Macromolecules: These are very large molecules, often composed of thousands of atoms. Proteins, DNA, and polysaccharides are examples of macromolecules crucial for life.
-
Materials: Materials are substances with specific physical and chemical properties, determined by their atomic and molecular structure and arrangement. These can range from simple elements to complex alloys and composites.
Conclusion: A Definitive Answer
In conclusion, a molecule is not smaller than an atom. Molecules are formed by the combination of two or more atoms chemically bonded together. The size of a molecule depends on the number and type of atoms it contains and their arrangement. Understanding the relationship between atoms and molecules is fundamental to grasping the structure and behavior of matter, impacting numerous scientific disciplines. While the visualization of these incredibly small entities can be challenging, understanding their fundamental characteristics is crucial for a solid grasp of the physical world.
Latest Posts
Latest Posts
-
Why Do Atoms Gain And Lose Electrons
Mar 28, 2025
-
Kinetic And Potential Energy In A Pendulum
Mar 28, 2025
-
Chapter 1 Solving Linear Equations Answers
Mar 28, 2025
-
In Which Carbohydrate Category Is Fructose Classified
Mar 28, 2025
-
What Are The Family Of Elements
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is A Molecule Smaller Than An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
