What Are The Family Of Elements
Muz Play
Mar 28, 2025 · 8 min read
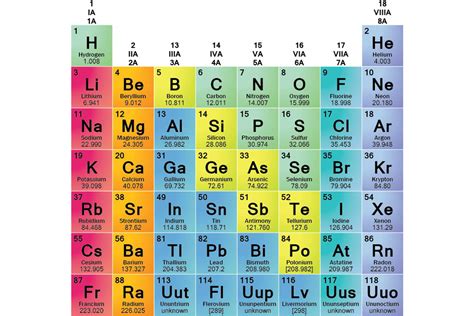
Table of Contents
What are the Families of Elements? A Deep Dive into the Periodic Table
The periodic table, that iconic chart adorning countless science classrooms, isn't just a random arrangement of elements. It's a meticulously organized system reflecting the fundamental properties and behaviors of matter. Understanding the families of elements, or groups, is key to unlocking the secrets of chemistry and predicting the properties of substances. This comprehensive guide delves into the diverse families of elements, exploring their characteristics, trends, and unique roles in the world around us.
The Power of the Periodic Table: Organization and Prediction
The periodic table's genius lies in its ability to predict an element's properties based solely on its position. Elements are arranged by increasing atomic number (the number of protons in their nucleus). More importantly, they are grouped into families or groups – vertical columns – sharing similar chemical properties due to having the same number of valence electrons. These valence electrons, located in the outermost shell, are the primary players in chemical bonding and reactions.
The horizontal rows are called periods, representing the filling of electron shells. Elements within the same period have electrons filling the same principal energy level, leading to variations in their properties across the period.
The Alkali Metals (Group 1): Highly Reactive and Soft
The alkali metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are incredibly reactive metals. Their single valence electron readily participates in chemical reactions, leading to the formation of +1 ions.
Key Characteristics of Alkali Metals:
- Low density: They are significantly lighter than other metals.
- Low melting and boiling points: They are relatively easy to melt and boil compared to other metals.
- Soft: They can be easily cut with a knife.
- Highly reactive: They readily react with water, producing hydrogen gas and heat, often explosively. Their reactivity increases down the group.
- Form ionic compounds: They readily lose their valence electron to form +1 ions, which bond strongly with non-metals.
Examples and Applications:
- Sodium (Na): Essential for human life, found in table salt (NaCl), and used in streetlights (sodium vapor lamps).
- Potassium (K): Vital for plant growth and essential for human nerve function.
- Lithium (Li): Used in batteries (lithium-ion batteries) and in some psychiatric medications.
The Alkaline Earth Metals (Group 2): Relatively Reactive, Stronger Metals
Group 2 elements, the alkaline earth metals – beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) – are less reactive than the alkali metals but still display significant reactivity. They have two valence electrons, leading to the formation of +2 ions.
Key Characteristics of Alkaline Earth Metals:
- Higher density than alkali metals: They are denser and harder than the alkali metals.
- Higher melting and boiling points than alkali metals: More energy is needed to overcome their stronger metallic bonds.
- Reactive, but less so than alkali metals: They react less vigorously with water and air.
- Form ionic compounds: They readily lose two valence electrons to form +2 ions.
Examples and Applications:
- Magnesium (Mg): Used in lightweight alloys (e.g., in aircraft), and in flares and fireworks.
- Calcium (Ca): Essential for strong bones and teeth, and used in cement and plaster.
- Beryllium (Be): Used in aerospace applications due to its lightweight and high strength.
The Transition Metals (Groups 3-12): A Diverse Group with Variable Oxidation States
The transition metals are a large and diverse group occupying the central block of the periodic table. Their defining feature is their ability to have multiple oxidation states (different numbers of electrons lost in forming ions), leading to a wide range of chemical properties and complex compounds.
Key Characteristics of Transition Metals:
- High melting and boiling points: They generally have strong metallic bonding.
- Good conductors of heat and electricity: Due to the presence of delocalized electrons.
- Variable oxidation states: They can lose different numbers of electrons, leading to a variety of compounds.
- Often form colored compounds: Their d-electrons can absorb and emit light at various wavelengths.
- Many are catalytic: They often speed up chemical reactions.
Examples and Applications:
- Iron (Fe): Essential for hemoglobin (oxygen transport in blood) and used in steel production.
- Copper (Cu): Excellent conductor of electricity, used in wiring and plumbing.
- Gold (Au): Highly prized for its inertness, malleability, and ductility.
- Platinum (Pt): Used as a catalyst in various industrial processes and in jewelry.
The Boron Family (Group 13): From Metals to Metalloids
The boron family, encompassing boron (B), aluminum (Al), gallium (Ga), indium (In), and thallium (Tl), showcases a transition from metalloid (boron) to metal properties down the group.
Key Characteristics of the Boron Family:
- Variety of properties: Boron is a metalloid, while the rest are metals.
- Three valence electrons: They tend to form +3 ions.
- Aluminum's importance: Aluminum is the most abundant metal in the Earth's crust and is widely used in various applications.
Examples and Applications:
- Aluminum (Al): Lightweight, strong, and corrosion-resistant; used in packaging, construction, and transportation.
- Boron (B): Used in glass, detergents, and insecticides.
The Carbon Family (Group 14): The Basis of Life and Semiconductors
The carbon family (carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb)) is crucial, with carbon being the cornerstone of life's organic molecules. Silicon is a key component of semiconductors.
Key Characteristics of the Carbon Family:
- Four valence electrons: They can form four bonds.
- Carbon's unique role: Carbon's ability to form long chains and rings leads to the immense diversity of organic compounds.
- Silicon's importance in technology: Silicon is a fundamental element in the semiconductor industry.
Examples and Applications:
- Carbon (C): Found in diamonds, graphite, and all organic compounds.
- Silicon (Si): Used in computer chips and solar cells.
The Nitrogen Family (Group 15): Essential Nutrients and Atmospheric Gases
The nitrogen family (nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi)) includes essential nutrients and elements with diverse applications.
Key Characteristics of the Nitrogen Family:
- Five valence electrons: They can form various bonds.
- Nitrogen's atmospheric dominance: Nitrogen gas makes up the majority of the Earth's atmosphere.
- Phosphorus's biological role: Phosphorus is vital for DNA, RNA, and ATP.
Examples and Applications:
- Nitrogen (N): Essential component of proteins and nucleic acids; used in fertilizers.
- Phosphorus (P): Crucial for plant and animal life; used in fertilizers and detergents.
The Oxygen Family (Group 16): Essential for Respiration and Reactive Non-metals
The oxygen family (oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po)) contains highly reactive non-metals, with oxygen being vital for respiration.
Key Characteristics of the Oxygen Family:
- Six valence electrons: They often form two bonds.
- Oxygen's crucial role: Oxygen is essential for respiration and combustion.
- Sulfur's importance: Sulfur is used in the production of sulfuric acid and vulcanized rubber.
Examples and Applications:
- Oxygen (O): Essential for respiration; used in medicine and welding.
- Sulfur (S): Used in sulfuric acid production and vulcanization of rubber.
The Halogens (Group 17): Highly Reactive Non-Metals
The halogens (fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)) are highly reactive non-metals with seven valence electrons, readily gaining one electron to form -1 ions.
Key Characteristics of Halogens:
- Seven valence electrons: They readily gain one electron to form -1 ions.
- Highly reactive: Their reactivity decreases down the group.
- Form diatomic molecules: They exist as diatomic molecules (e.g., F2, Cl2).
Examples and Applications:
- Chlorine (Cl): Used in water purification and as a disinfectant.
- Fluorine (F): Used in toothpaste to prevent tooth decay.
- Iodine (I): Essential for thyroid hormone production.
The Noble Gases (Group 18): Inert and Unreactive
The noble gases (helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)) are characterized by their extreme inertness. Their full valence electron shells make them very stable and unreactive.
Key Characteristics of Noble Gases:
- Full valence electron shells: They have eight valence electrons (except helium, which has two).
- Inert: They rarely participate in chemical reactions.
- Used in lighting: Neon and other noble gases are used in various lighting applications.
Examples and Applications:
- Helium (He): Used in balloons and MRI machines.
- Neon (Ne): Used in neon signs.
- Argon (Ar): Used in welding and as a protective atmosphere.
Beyond the Main Groups: Lanthanides and Actinides
The lanthanides and actinides, located at the bottom of the periodic table, are f-block elements with unique electronic configurations and properties. They are often grouped together due to their similar chemical properties.
Key Characteristics of Lanthanides and Actinides:
- f-block elements: They have electrons filling the f-subshell.
- Similar chemical properties: Due to their similar electronic configurations.
- Radioactivity: Many actinides are radioactive.
Examples and Applications:
- Lanthanides: Used in magnets and catalysts.
- Actinides: Used in nuclear reactors and weapons.
Conclusion: The Periodic Table – A Window into the Nature of Matter
The periodic table, with its organized families of elements, provides a powerful framework for understanding the properties and behaviors of matter. By studying the trends within each group and across periods, we gain insights into chemical reactivity, bonding, and the diverse roles of elements in the natural world and our technologies. From the highly reactive alkali metals to the inert noble gases, each family offers unique characteristics and applications, highlighting the fascinating complexity of the chemical world. The periodic table remains a fundamental tool for chemists, researchers, and anyone seeking a deeper understanding of the elements that build our universe.
Latest Posts
Latest Posts
-
Which Statement Describes The Citric Acid Cycle
Mar 31, 2025
-
Why Do Plants Love Water In Bio Terms
Mar 31, 2025
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
-
Why Does Km Increase In Competitive Inhibition
Mar 31, 2025
-
What Is The Electron Configuration Of Beryllium
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are The Family Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
