Freezing Point Depression Constant Of Water
Muz Play
Mar 16, 2025 · 6 min read
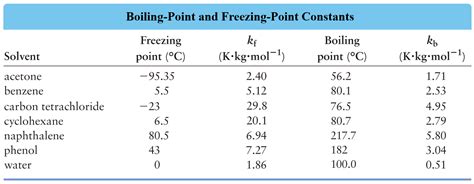
Table of Contents
Freezing Point Depression Constant of Water: A Deep Dive
The freezing point depression constant, often denoted as K<sub>f</sub>, is a fundamental colligative property of solvents. This means it depends solely on the concentration of solute particles, not their identity. Understanding K<sub>f</sub> for water is crucial in various fields, from chemistry and cryobiology to environmental science and food processing. This comprehensive guide delves into the intricacies of water's freezing point depression constant, exploring its definition, calculation, applications, and limitations.
What is the Freezing Point Depression Constant?
The freezing point of a pure solvent, like water, is the temperature at which the liquid and solid phases are in equilibrium. Introducing a solute into this solvent lowers the freezing point. This phenomenon, known as freezing point depression, is a direct consequence of the disruption of the solvent's crystalline structure by the solute particles. The extent of this depression is directly proportional to the molality (moles of solute per kilogram of solvent) of the solution and is described by the following equation:
ΔT<sub>f</sub> = K<sub>f</sub> * m * i
Where:
- ΔT<sub>f</sub> is the freezing point depression (in °C or K) – the difference between the freezing point of the pure solvent and the freezing point of the solution.
- K<sub>f</sub> is the cryoscopic constant or freezing point depression constant (in °C kg/mol or K kg/mol) – a characteristic property of the solvent. For water, K<sub>f</sub> = 1.86 °C kg/mol.
- m is the molality of the solute (mol/kg).
- i is the van't Hoff factor – accounts for the dissociation of the solute into ions. For non-electrolytes (substances that don't dissociate in solution), i = 1. For strong electrolytes, i is approximately equal to the number of ions produced per formula unit. For weak electrolytes, i is between 1 and the number of ions produced, depending on the degree of dissociation.
Understanding the Value of K<sub>f</sub> for Water (1.86 °C kg/mol)
The value of 1.86 °C kg/mol for water's K<sub>f</sub> is experimentally determined. It represents the freezing point depression caused by dissolving one mole of a non-volatile, non-electrolyte solute in one kilogram of water. This value reflects the specific interactions between water molecules and the disruption caused by the introduction of foreign particles.
The relatively high value of K<sub>f</sub> for water compared to some other solvents highlights water's strong intermolecular forces (hydrogen bonding). These strong forces contribute to the relatively high melting point of pure water, and their disruption requires a significant change in temperature to compensate.
Factors Affecting K<sub>f</sub>
While K<sub>f</sub> is primarily a property of the solvent, subtle variations can occur due to:
- Pressure: Changes in pressure can slightly affect the freezing point, and consequently, the K<sub>f</sub> value. However, at standard pressure, the effect is negligible.
- Solvent Purity: Impurities in the solvent can alter its freezing point and affect the measured K<sub>f</sub>. High-purity water is essential for accurate measurements.
- Intermolecular Interactions: The strength of intermolecular interactions between solute and solvent molecules influences the freezing point depression. Stronger interactions can lead to deviations from the ideal behavior predicted by the equation.
Calculating Freezing Point Depression
Let's illustrate the calculation with an example. Suppose we dissolve 58.5 grams of NaCl (sodium chloride, molar mass = 58.5 g/mol) in 1 kg of water. NaCl is a strong electrolyte that dissociates into two ions (Na<sup>+</sup> and Cl<sup>-</sup>) in water, so i ≈ 2.
-
Calculate the molality (m): m = (moles of solute) / (kg of solvent) = (58.5 g / 58.5 g/mol) / (1 kg) = 1 mol/kg
-
Apply the freezing point depression equation: ΔT<sub>f</sub> = K<sub>f</sub> * m * i = (1.86 °C kg/mol) * (1 mol/kg) * 2 = 3.72 °C
-
Determine the freezing point of the solution: The freezing point of pure water is 0 °C. Therefore, the freezing point of the NaCl solution is 0 °C - 3.72 °C = -3.72 °C.
Applications of Freezing Point Depression Constant of Water
The understanding and application of the freezing point depression constant of water are widespread across various disciplines:
1. Cryobiology:
- Cryopreservation: Freezing point depression is crucial in cryopreservation, the process of preserving biological materials at ultra-low temperatures. Cryoprotective agents (CPAs) are added to reduce the formation of ice crystals, which can damage cells and tissues. The choice and concentration of CPAs are determined, in part, by their effect on the freezing point.
2. Road De-icing:
- Salt Application: Salt (NaCl or CaCl<sub>2</sub>) is commonly used to de-ice roads and pavements during winter. The salt dissolves in the snow or ice, lowering the freezing point and causing melting. The effectiveness of de-icing depends on factors like the salt concentration and ambient temperature.
3. Food Preservation:
- Freezing Foods: Freezing food to preserve it leverages the principles of freezing point depression. While freezing slows down bacterial growth, the effectiveness depends on the freezing rate and the freezing point of the food itself. The addition of solutes to some food products can alter their freezing point and improve preservation.
4. Chemical Analysis:
- Determining Molar Mass: The freezing point depression can be used to determine the molar mass of an unknown solute. By measuring the freezing point depression of a solution of known concentration, the molar mass can be calculated using the freezing point depression equation.
5. Environmental Science:
- Salinity Measurement: The freezing point of water can be used to estimate the salinity of water bodies. Higher salinity results in a lower freezing point. This is relevant in understanding the behavior of aquatic ecosystems and for monitoring water quality.
Limitations and Deviations from Ideality
While the freezing point depression equation provides a good approximation for dilute solutions, deviations can occur in concentrated solutions or when dealing with strong electrolytes. These deviations arise from several factors:
-
Ion Pairing: In concentrated solutions of electrolytes, ions can interact with each other, reducing the effective number of particles and lowering the freezing point depression compared to the ideal value.
-
Non-Ideal Solutions: In non-ideal solutions, the interactions between solute and solvent molecules are not as simple as assumed in the ideal model. These interactions can either enhance or diminish the freezing point depression.
-
Activity Coefficients: To account for deviations from ideality, activity coefficients can be incorporated into the freezing point depression equation. Activity coefficients correct for the non-ideal behavior of the solution.
Conclusion
The freezing point depression constant of water is a vital parameter with numerous applications across diverse fields. Understanding its value (1.86 °C kg/mol) and the factors that influence it is crucial for accurately predicting the freezing point of aqueous solutions and for designing processes and applications that leverage this colligative property. While the simple equation provides a valuable starting point, the limitations and deviations from ideality must be considered for accurate predictions and analysis, particularly in concentrated solutions or with strong electrolytes. Further research and refinement of the models are ongoing to improve the accuracy and predictive power of freezing point depression calculations in diverse and complex systems. The continued exploration and understanding of this constant will undoubtedly lead to advancements in various scientific and technological domains.
Latest Posts
Latest Posts
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
-
How Do I Solve Square Root Equations
Mar 17, 2025
-
What Is A Monomer Of A Nucleic Acid
Mar 17, 2025
-
100 Most Important People Of The Century
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Depression Constant Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
