Gay Lussac's Law Of Combining Volumes
Muz Play
Mar 17, 2025 · 5 min read
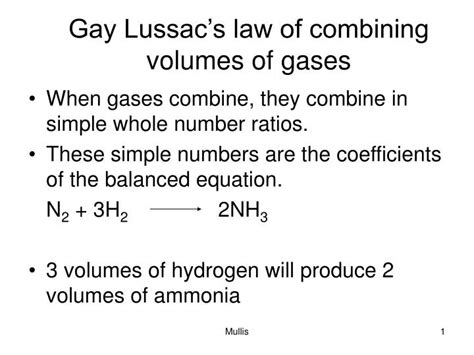
Table of Contents
Gay-Lussac's Law of Combining Volumes: A Deep Dive into Gas Reactions
Gay-Lussac's Law of Combining Volumes, a cornerstone of chemistry, elegantly describes the relationships between the volumes of gases involved in chemical reactions. While seemingly simple at first glance, its implications are profound, paving the way for a deeper understanding of the atomic nature of matter and the quantitative aspects of chemical processes. This comprehensive guide will delve into the law itself, its historical context, experimental verification, limitations, and its crucial role in the development of modern chemistry.
Understanding Gay-Lussac's Law
Gay-Lussac's Law of Combining Volumes, also known as Gay-Lussac's Law of Combining Gases, states that when gases react, they do so in volumes that bear a simple, whole-number ratio to each other and to the volumes of the gaseous products, provided that the temperature and pressure remain constant. This simple statement revolutionized the understanding of gas reactions. Before its articulation, chemical reactions were largely treated as qualitative observations, lacking the precise, quantitative relationships that Gay-Lussac's law provided.
The essence of the law lies in the simple whole-number ratio. This observation is not arbitrary; it reflects the fundamental nature of atoms and molecules and how they combine to form new substances. The whole-number ratios directly correlate with the stoichiometric coefficients in balanced chemical equations, signifying the relative number of molecules reacting and being produced.
Example: The Reaction of Hydrogen and Oxygen
Consider the reaction between hydrogen and oxygen to form water:
2H₂(g) + O₂(g) → 2H₂O(l)
According to Gay-Lussac's Law, the volumes of hydrogen and oxygen reacting will be in a simple ratio of 2:1. If we measure the volumes of hydrogen and oxygen reacting at constant temperature and pressure, we find that two volumes of hydrogen always react with one volume of oxygen to produce (initially) two volumes of water vapor (before condensation to liquid). This simple ratio of 2:1:2 perfectly aligns with the stoichiometric coefficients in the balanced chemical equation.
Historical Context and Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (1778-1850), a prominent French chemist and physicist, meticulously conducted experiments on gas reactions. His observations, published in 1808, laid the foundation for Gay-Lussac's Law. Before Gay-Lussac's work, the quantitative relationships between gas volumes in chemical reactions were largely unknown. Scientists focused primarily on mass relationships, as elucidated by the Law of Conservation of Mass.
Gay-Lussac's contributions weren't limited to this law. His scientific legacy includes significant work on gas expansion with temperature (Charles's Law), the development of techniques for gas analysis, and extensive studies in meteorology and physics.
Experimental Verification and Implications
Numerous experiments have verified Gay-Lussac's Law's accuracy under controlled conditions of constant temperature and pressure. Precise volumetric measurements of gases reacting, using specialized glassware, consistently demonstrate the simple whole-number ratios predicted by the law. This experimental validation underscores the law's importance in establishing the quantitative foundations of chemistry.
The implications of Gay-Lussac's Law are far-reaching:
-
Atomic Theory Reinforcement: The law strongly supports Dalton's atomic theory. The simple whole-number ratios of reacting gas volumes imply that gases consist of discrete particles (atoms or molecules) that combine in simple whole-number ratios. This provided crucial experimental evidence for the atomic theory which at that point was still under development.
-
Avogadro's Hypothesis: Amedeo Avogadro, building on Gay-Lussac's work, proposed his hypothesis in 1811. Avogadro's hypothesis states that equal volumes of all gases at the same temperature and pressure contain the same number of molecules. This hypothesis elegantly explained the simple whole-number ratios observed by Gay-Lussac. Avogadro's hypothesis provided the missing link to interpret the gas volume ratios in terms of molecular ratios.
-
Stoichiometric Calculations: Gay-Lussac's Law is fundamental to stoichiometric calculations. It allows chemists to predict the volumes of reactants and products involved in a gas-phase reaction, given the balanced chemical equation and the volume of one of the reactants or products.
Limitations of Gay-Lussac's Law
While powerful and widely applicable, Gay-Lussac's Law has some limitations:
-
Ideal Gas Behavior: The law holds true only for gases that behave ideally. At high pressures or low temperatures, real gases deviate from ideal behavior, and the simple whole-number ratios may not be strictly observed. Real gases have intermolecular forces and molecular volumes, unlike an ideal gas that doesn't consider these factors.
-
Gaseous Reactants and Products: Gay-Lussac's Law directly applies only to reactions involving gases. It doesn't directly address reactions involving liquids or solids.
-
Constant Temperature and Pressure: The law is valid only when the temperature and pressure remain constant throughout the reaction. Any changes in temperature or pressure will affect the gas volumes and invalidate the simple whole-number ratios.
Gay-Lussac's Law and Modern Chemistry
Gay-Lussac's Law remains a cornerstone of modern chemistry. Its principle of simple whole-number ratios for reacting gas volumes is integral to understanding stoichiometry, chemical equations, and the quantitative relationships between reactants and products.
The law’s importance extends beyond basic stoichiometry. It's crucial for various applications in:
-
Industrial Chemistry: In industrial processes involving gases (like ammonia synthesis or combustion reactions), Gay-Lussac's law helps optimize reaction conditions and predict yields.
-
Environmental Science: Understanding gas-phase reactions is vital for assessing air pollution and modelling atmospheric processes. Gay-Lussac's law provides a basis for these quantitative analyses.
-
Analytical Chemistry: Quantitative gas analysis relies heavily on the principles of Gay-Lussac's law. Volumetric analysis techniques utilize this law to determine the concentration of unknown gas samples.
Conclusion: A Lasting Legacy
Gay-Lussac's Law of Combining Volumes represents a pivotal moment in the development of chemistry. It transitioned the field from primarily qualitative observations to precise, quantitative predictions about gas reactions. While it has limitations concerning ideal gas behavior and specific reaction conditions, its enduring legacy lies in its fundamental contribution to atomic theory, Avogadro's hypothesis, stoichiometry, and numerous applications across various scientific disciplines. The simple, elegant relationship between gas volumes in chemical reactions, as described by Gay-Lussac, continues to be a powerful tool for understanding the molecular world. Its profound impact on the advancement of chemical science is undeniable and ensures its relevance in modern chemistry and beyond.
Latest Posts
Latest Posts
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Gay Lussac's Law Of Combining Volumes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
