Ground State Energy Of Hydrogen Atom
Muz Play
Mar 22, 2025 · 6 min read
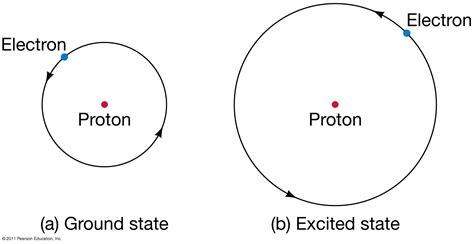
Table of Contents
Ground State Energy of the Hydrogen Atom: A Deep Dive
The hydrogen atom, the simplest atom in the universe, serves as a foundational cornerstone in our understanding of quantum mechanics. Its relative simplicity allows for precise calculations and provides a crucial stepping stone towards comprehending more complex atomic structures. A key concept within this understanding is the ground state energy, the lowest energy level that the electron can occupy within the hydrogen atom. This article delves deep into the calculation, significance, and implications of this fundamental energy level.
Understanding the Bohr Model and its Limitations
Before diving into the quantum mechanical approach, it's helpful to briefly revisit the Bohr model, a historical precursor that, while inaccurate, provides intuitive context. The Bohr model postulates that the electron orbits the nucleus in specific, quantized energy levels. The ground state in this model is the orbit closest to the nucleus, possessing the lowest energy. While this model correctly predicts the ground state energy (to a reasonable approximation), it fails to accurately describe the behavior of electrons and fails to account for the observed spectra of more complex atoms.
The limitations of the Bohr model include:
- Incorrect electron behavior: It depicts electrons as orbiting the nucleus in well-defined paths, a classical picture that contradicts the wave-particle duality of electrons.
- Inadequacy for multi-electron atoms: The model struggles to predict the energy levels and behavior of electrons in atoms with more than one electron due to electron-electron interactions.
- Failure to explain fine structure: It cannot explain the subtle splitting of spectral lines observed under high resolution.
The Quantum Mechanical Approach: Schrödinger Equation and the Hydrogen Atom
The accurate description of the hydrogen atom's ground state energy requires the application of quantum mechanics, specifically the time-independent Schrödinger equation. This equation describes the electron's wave function, which determines the probability of finding the electron at a particular location around the nucleus. For the hydrogen atom, the Schrödinger equation is a three-dimensional partial differential equation that takes into account the Coulombic interaction between the positively charged proton and the negatively charged electron.
Solving the Schrödinger Equation
Solving the Schrödinger equation for the hydrogen atom yields a set of solutions, each corresponding to a specific energy level and wave function. The ground state corresponds to the lowest energy solution, and its wave function is denoted as ψ₁₀₀ (n=1, l=0, m=0). The quantum numbers (n, l, m) represent the principal quantum number, the azimuthal quantum number, and the magnetic quantum number, respectively.
- Principal Quantum Number (n): Determines the energy level and size of the orbital (n = 1, 2, 3...). The ground state has n=1.
- Azimuthal Quantum Number (l): Determines the shape of the orbital (l = 0, 1, 2...n-1). The ground state has l=0, corresponding to an s orbital.
- Magnetic Quantum Number (m): Determines the orientation of the orbital in space (m = -l, -l+1...0...l-1, l). The ground state has m=0, meaning the s orbital is spherically symmetric.
Calculating the Ground State Energy
The solution to the Schrödinger equation for the hydrogen atom provides the ground state energy, E₁, as:
E₁ = -13.6 eV
This value, -13.6 electron volts (eV), represents the energy required to completely ionize the hydrogen atom from its ground state (i.e., to remove the electron completely). The negative sign indicates that the electron is bound to the nucleus; it requires energy input to free it.
Derivation of the Formula (Simplified)
A detailed derivation of this formula requires advanced mathematical techniques involving solving the differential equation. However, a simplified explanation can be provided: The energy is determined by the balance between the kinetic energy of the electron and the potential energy of the electron-proton interaction. The quantization of angular momentum (a consequence of the wave-like nature of the electron) restricts the possible energy levels to discrete values. The lowest of these values is the ground state energy.
Factors Affecting Ground State Energy
The ground state energy of the hydrogen atom is primarily determined by:
- Coulomb's Constant (k): Describes the strength of the electrostatic interaction between the proton and the electron.
- Electron Mass (mₑ): The mass of the electron influences its kinetic energy.
- Planck's Constant (h): A fundamental constant in quantum mechanics that quantizes energy and angular momentum.
- Reduced Mass (μ): In a more accurate calculation, the reduced mass of the electron-proton system is used instead of the electron mass alone. This accounts for the slight motion of the proton.
Significance and Implications of the Ground State Energy
The ground state energy of the hydrogen atom holds immense significance in various fields of physics and chemistry:
- Atomic Spectroscopy: The energy difference between the ground state and higher energy levels determines the wavelengths of light emitted or absorbed during electronic transitions. This forms the basis of atomic spectroscopy, a powerful tool for identifying and analyzing different elements.
- Chemical Bonding: The ground state energy dictates the stability of the hydrogen atom and its tendency to form chemical bonds with other atoms. The energy released or absorbed during bond formation is directly related to the energy levels involved.
- Laser Technology: Understanding the energy levels of the hydrogen atom is crucial in developing and improving laser technologies that utilize the transitions between these levels.
- Astrophysics: The hydrogen atom is the most abundant element in the universe, and its ground state energy plays a critical role in understanding stellar evolution, the formation of galaxies, and other astrophysical phenomena. Emission and absorption lines from hydrogen are observed in the spectra of stars and nebulae.
- Quantum Computing: The behavior of electrons in atoms, especially hydrogen's relatively simple structure, is important for understanding and developing novel quantum computing technologies.
Beyond the Hydrogen Atom: Multi-electron Atoms and Molecules
While the hydrogen atom provides a fundamental understanding of atomic structure and energy levels, more complex atoms and molecules pose significant challenges. The presence of multiple electrons introduces electron-electron interactions that complicate the Schrödinger equation significantly. Approximations and numerical methods are often required to solve these equations and calculate energy levels. However, the principles learned from the hydrogen atom form the basis for understanding these more complex systems. Concepts like shielding and penetration of orbitals, resulting from the interactions between electrons, significantly affect the energy levels of electrons in multi-electron atoms.
Conclusion: A Foundation for Quantum Mechanics
The ground state energy of the hydrogen atom serves as a critical benchmark in our understanding of quantum mechanics. Its precise calculation, grounded in the Schrödinger equation, provides a solid foundation for exploring the complex world of atomic and molecular structure. While the simplicity of the hydrogen atom contrasts with the complexity of many other systems, its study offers essential insights into the fundamental principles that govern the behavior of matter at the atomic level. The implications of this seemingly simple calculation extend across numerous scientific disciplines, highlighting the profound impact of basic quantum mechanics on our understanding of the universe. Furthermore, continued research into atomic structure, fueled by a strong foundation in basic atomic theory, is crucial for ongoing advancements in fields such as materials science, nanotechnology, and energy technologies. The journey to understanding the complexities of the universe begins with the simplicity of the hydrogen atom.
Latest Posts
Latest Posts
-
How Does A Buffer Solution Resist A Change In Ph
Mar 24, 2025
-
Which Of The Following Are Chemical Reactions
Mar 24, 2025
-
Do Strong Acids Have High Or Low Ka
Mar 24, 2025
-
What Kind Of Waves Do Televisions Use
Mar 24, 2025
-
Where Is Most Of The Mass Of An Atom Located
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Ground State Energy Of Hydrogen Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
