How Does A Buffer Solution Resist A Change In Ph
Muz Play
Mar 24, 2025 · 6 min read
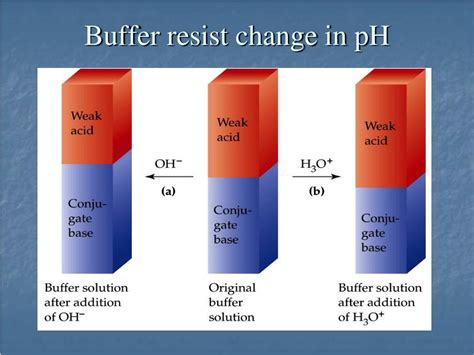
Table of Contents
How Does a Buffer Solution Resist a Change in pH?
Maintaining a stable pH is crucial in numerous chemical and biological systems. From the precise pH regulation within our bodies to the optimal conditions required for many industrial chemical processes, the ability to resist pH changes is paramount. This is where buffer solutions come in. This comprehensive guide delves deep into the mechanism behind a buffer solution's remarkable ability to resist changes in pH, explaining the chemistry, the types of buffers, and their widespread applications.
Understanding pH and its Importance
Before diving into buffer solutions, let's establish a solid understanding of pH itself. pH is a measure of the acidity or alkalinity of a solution, expressed on a scale from 0 to 14. A pH of 7 is considered neutral, values below 7 are acidic, and values above 7 are alkaline (basic). The pH scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion (H⁺) concentration.
The concentration of H⁺ ions dictates the acidity of a solution. A high concentration of H⁺ ions signifies a strong acid, while a low concentration indicates a weak acid or a basic solution. Maintaining a specific pH is crucial because many chemical reactions and biological processes are highly sensitive to pH changes. Even slight deviations can significantly impact reaction rates, enzyme activity, and overall system stability.
What is a Buffer Solution?
A buffer solution is an aqueous solution that resists changes in pH upon the addition of small amounts of acid or base. This remarkable property stems from its unique composition: a buffer typically consists of a weak acid and its conjugate base (or a weak base and its conjugate acid). This combination allows the buffer to neutralize both added acids and bases, thereby minimizing the impact on the overall pH.
Key Characteristics of Buffer Solutions:
- Resistance to pH change: This is the defining characteristic, enabling them to maintain a relatively constant pH even when subjected to external influences.
- Weak acid/base and conjugate pair: The presence of both a weak acid and its conjugate base (or vice versa) is essential for buffering capacity.
- Effective within a specific pH range: Each buffer has an optimal pH range within which it effectively resists changes. This range is determined by the pKa (acid dissociation constant) of the weak acid component.
The Chemistry Behind Buffer Action: The Henderson-Hasselbalch Equation
The behavior of a buffer solution is elegantly described by the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
where:
- pH is the pH of the buffer solution
- pKa is the negative logarithm of the acid dissociation constant (Ka) of the weak acid
- [A⁻] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
This equation reveals the crucial interplay between the weak acid, its conjugate base, and the resulting pH. When the concentrations of the weak acid and its conjugate base are equal ([A⁻] = [HA]), the pH of the buffer solution equals the pKa of the weak acid. This represents the buffer's optimal buffering capacity.
How the Buffer Works: A Step-by-Step Explanation
Let's consider a buffer solution containing acetic acid (CH₃COOH) and its conjugate base, acetate ion (CH₃COO⁻).
-
Addition of a Strong Acid (e.g., HCl): When a strong acid like HCl is added, it donates H⁺ ions. These H⁺ ions are readily consumed by the acetate ions (CH₃COO⁻) to form acetic acid (CH₃COOH). This reaction minimizes the increase in H⁺ concentration, thus preventing a significant drop in pH.
CH₃COO⁻ + H⁺ ⇌ CH₃COOH
-
Addition of a Strong Base (e.g., NaOH): When a strong base like NaOH is added, it donates OH⁻ ions. These OH⁻ ions react with the acetic acid (CH₃COOH) to form water (H₂O) and acetate ions (CH₃COO⁻). This reaction minimizes the increase in OH⁻ concentration, preventing a significant rise in pH.
CH₃COOH + OH⁻ ⇌ CH₃COO⁻ + H₂O
In both cases, the buffer solution effectively neutralizes the added acid or base, preventing large changes in pH. The equilibrium shifts to consume the added H⁺ or OH⁻ ions, maintaining a relatively constant pH within its buffering range.
Types of Buffer Solutions
Various buffer systems exist, each suited for different pH ranges and applications. Some common examples include:
-
Phosphate buffers: These are widely used in biological systems because they are compatible with living organisms and operate within a physiological pH range. Common phosphate buffers include mixtures of monobasic sodium phosphate (NaH₂PO₄) and dibasic sodium phosphate (Na₂HPO₄).
-
Acetate buffers: These are often employed in chemical reactions and analytical procedures requiring a slightly acidic pH. They typically involve acetic acid (CH₃COOH) and its sodium salt, sodium acetate (CH₃COONa).
-
Citrate buffers: Citric acid and its salts form another effective buffering system, useful in a slightly acidic pH range. Citrate buffers are often used in food and beverage applications.
-
Tris buffers (Tris(hydroxymethyl)aminomethane): These are popular in biochemistry and molecular biology, often used for biological experiments involving proteins and enzymes.
The choice of buffer depends on the specific pH range needed and compatibility with the system or reaction under study.
Buffer Capacity and its Limitations
While buffer solutions excel at resisting pH changes, their capacity is not unlimited. Buffer capacity refers to the amount of acid or base a buffer can neutralize before its pH changes significantly. The buffer capacity is influenced by:
- The concentrations of the weak acid and conjugate base: Higher concentrations lead to greater buffer capacity.
- The ratio of weak acid to conjugate base: A buffer is most effective when the ratio is close to 1:1 (pH ≈ pKa).
- The strength of the weak acid: A weaker acid will have a smaller buffer range.
Once the buffer capacity is exceeded, the pH will change dramatically with the addition of further acid or base. This signifies that the buffer is exhausted and no longer effectively resists pH changes.
Applications of Buffer Solutions
Buffer solutions play a pivotal role in various fields, including:
-
Biological Systems: Maintaining a stable pH is crucial for the proper functioning of enzymes and other biological molecules. Buffers like phosphate buffers play a vital role in maintaining the pH of blood and other bodily fluids.
-
Medicine: Many pharmaceutical formulations utilize buffers to ensure drug stability and effectiveness. Buffers help maintain the desired pH for injections and other drug delivery systems.
-
Analytical Chemistry: Buffer solutions are essential in many analytical techniques, such as titrations, electrophoresis, and chromatography. They help control the pH to optimize the analytical process.
-
Industrial Processes: Buffer solutions are employed in various industrial processes to control pH, ensuring optimal reaction conditions and product quality. Examples include food processing, textile manufacturing, and water treatment.
-
Environmental Science: Buffers play a role in maintaining the pH of aquatic ecosystems, mitigating the effects of acid rain and pollution.
Conclusion
Buffer solutions are indispensable for maintaining stable pH in numerous scientific, industrial, and biological applications. Their ability to resist pH changes stems from the equilibrium between a weak acid and its conjugate base (or vice versa). The Henderson-Hasselbalch equation provides a quantitative understanding of this equilibrium and the factors affecting buffer capacity. While buffer capacity is not unlimited, understanding its limitations and choosing the appropriate buffer system for a given application is vital for achieving the desired pH control and system stability. This detailed examination highlights the fundamental principles behind buffer solutions and underscores their widespread importance across diverse fields.
Latest Posts
Latest Posts
-
What Stage Of Cellular Respiration Produces The Most Atp
Mar 25, 2025
-
Which Correctly Summarizes The Trend In Electron Affinity
Mar 25, 2025
-
Delta H Delta S Delta G Chart
Mar 25, 2025
-
A Chemical Equation Is Balanced When
Mar 25, 2025
-
What Is The Average Kinetic Energy
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Does A Buffer Solution Resist A Change In Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
