Which Of The Following Are Chemical Reactions
Muz Play
Mar 24, 2025 · 6 min read
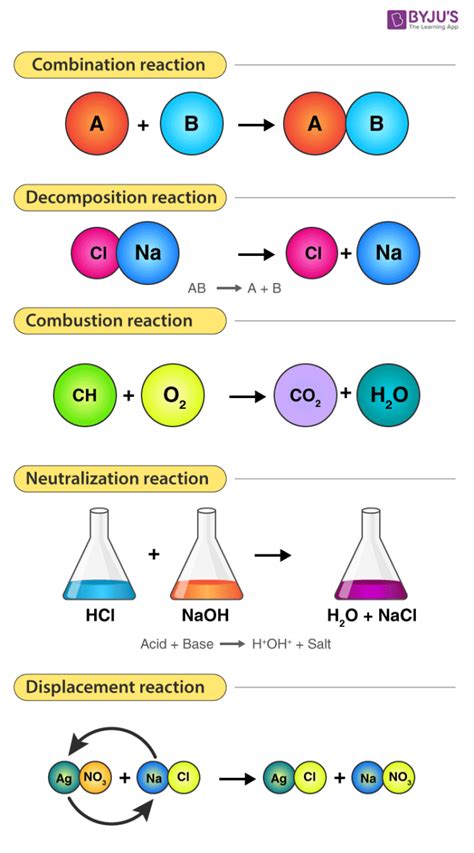
Table of Contents
Which of the Following Are Chemical Reactions? A Comprehensive Guide
Identifying chemical reactions from a list of processes can seem daunting, but understanding the key characteristics that define a chemical change makes it much simpler. This comprehensive guide will delve into the fundamental principles of chemical reactions, providing clear examples and explanations to help you confidently distinguish between chemical and physical changes. We'll explore various scenarios and equip you with the tools to effectively identify chemical reactions in any context.
Understanding Chemical Reactions
A chemical reaction, also known as a chemical change, is a process that leads to the transformation of one or more substances into one or more new substances with different chemical properties. Crucially, this transformation involves the rearrangement of atoms and the breaking and forming of chemical bonds. The original substances are called reactants, and the new substances formed are called products.
Several key indicators can help you identify a chemical reaction:
Key Indicators of Chemical Reactions:
-
Formation of a precipitate: A precipitate is a solid that forms from a solution during a chemical reaction. This is a clear visual indication of a chemical change. For example, when you mix solutions of lead(II) nitrate and potassium iodide, a yellow precipitate of lead(II) iodide forms.
-
Evolution of a gas: The production of a gas, often observable as bubbles or effervescence, signals a chemical reaction. Baking soda reacting with vinegar is a classic example, producing carbon dioxide gas.
-
Change in temperature: Many chemical reactions either release heat (exothermic reactions) or absorb heat (endothermic reactions). A significant temperature change, either an increase or decrease, is a strong indicator of a chemical reaction.
-
Change in color: A change in color often accompanies a chemical reaction, as the products have different light-absorbing properties than the reactants. For instance, the rusting of iron involves a color change from silvery-gray to reddish-brown.
-
Change in odor: The formation of new substances with different volatile components can lead to a noticeable change in odor. Spoiling food is a prime example of a chemical reaction accompanied by a change in smell.
-
Irreversibility (in many cases): While some chemical reactions are reversible, many are not. Once a chemical reaction has occurred, it often requires another chemical reaction to reverse the process. This irreversibility is a key distinguishing feature from physical changes.
Differentiating Chemical Reactions from Physical Changes
It's crucial to distinguish chemical reactions from physical changes. Physical changes alter the form or appearance of a substance without changing its chemical composition. Examples include changes in state (melting, boiling, freezing), dissolving, and crushing. No new substances are formed during a physical change.
Here's a table summarizing the key differences:
| Feature | Chemical Change (Reaction) | Physical Change |
|---|---|---|
| Composition | Changes; new substances are formed | Remains the same; no new substances are formed |
| Bonds | Bonds are broken and new bonds are formed | Bonds are not broken or formed |
| Properties | Chemical properties change; new properties emerge | Chemical properties remain the same |
| Reversibility | Often irreversible or difficult to reverse | Usually reversible |
| Examples | Burning, rusting, digestion, cooking, fermentation | Melting ice, dissolving sugar, breaking a glass |
Examples: Identifying Chemical Reactions
Let's examine several scenarios and determine if they represent chemical reactions:
Scenario 1: Burning Wood
Burning wood is undoubtedly a chemical reaction. Wood (primarily cellulose) reacts with oxygen in the air, producing carbon dioxide, water vapor, ash, and heat. This involves the breaking of bonds in the cellulose molecules and the formation of new bonds in the products. The change is irreversible, and significant heat is released (exothermic reaction). There's also a dramatic color change and evolution of gases.
Scenario 2: Melting Ice
Melting ice is a physical change. Ice (solid water) transforms into liquid water, but the chemical composition remains the same – H₂O. No new substances are formed, and the change is reversible. The only change is the state of matter.
Scenario 3: Dissolving Salt in Water
Dissolving salt (NaCl) in water is a physical change. The salt crystals break down into individual ions (Na⁺ and Cl⁻) surrounded by water molecules, but the chemical composition of the salt remains unchanged. The salt can be recovered by evaporating the water. While there is an apparent change (solid to dissolved ions), it's still a physical process.
Scenario 4: Baking a Cake
Baking a cake is a complex process involving several chemical reactions. The baking powder (a mixture of baking soda and an acid) reacts with moisture and heat to produce carbon dioxide gas, causing the cake to rise. The flour proteins denature and undergo complex chemical changes during baking, resulting in a structural change. The color and texture also significantly change during baking.
Scenario 5: Rusting of Iron
Rusting is a chemical reaction known as oxidation. Iron reacts with oxygen and water to form iron(III) oxide (rust). This involves a change in chemical composition, a change in color (from silvery gray to reddish-brown), and is irreversible under normal conditions.
Scenario 6: Mixing Sand and Water
Mixing sand and water is a physical change. The sand particles are suspended in the water, but they retain their original chemical composition. Separating the sand from the water is easily done through simple filtration, demonstrating the reversibility of the change.
Scenario 7: Photosynthesis
Photosynthesis is a crucial chemical reaction occurring in plants. Plants use sunlight, carbon dioxide, and water to produce glucose (a sugar) and oxygen. This involves a complex series of chemical reactions and a significant change in chemical composition.
Scenario 8: Digestion
Digestion is a series of chemical reactions. Enzymes break down complex food molecules (carbohydrates, proteins, fats) into simpler molecules that can be absorbed by the body. This involves numerous enzymatic reactions, resulting in a significant change in the chemical composition of the food.
Scenario 9: Electrolysis of Water
Electrolysis of water is a chemical reaction. Passing an electric current through water decomposes it into hydrogen and oxygen gases. This is a chemical change since new substances (hydrogen and oxygen) are formed from the decomposition of water.
Scenario 10: Mixing Oil and Water
Mixing oil and water results in a physical change. Oil and water are immiscible; they do not mix, forming distinct layers. No chemical reaction occurs, and the original substances remain unchanged.
Advanced Considerations: Recognizing Complex Reactions
Some scenarios might appear ambiguous at first glance. For example, dissolving some substances in water can cause a temperature change, seemingly indicating a chemical reaction. However, if the substance simply dissolves without forming new chemical species, it’s still a physical change (although it can be an exothermic or endothermic process). Careful observation and a deeper understanding of the involved substances are key to accurate classification.
Conclusion
Identifying chemical reactions requires a thorough understanding of the fundamental principles of chemical change and the key indicators that distinguish them from physical changes. By paying close attention to indicators like precipitate formation, gas evolution, temperature changes, color changes, odor changes, and irreversibility, one can confidently differentiate between these two types of changes. Remember to carefully consider the specific substances involved and the nature of the transformation to accurately classify a process as a chemical reaction or a physical change. This guide provides a comprehensive framework to enhance your ability to analyze and identify chemical reactions in various contexts. Through consistent practice and a deeper understanding of chemical principles, identifying chemical reactions becomes increasingly straightforward.
Latest Posts
Latest Posts
-
Delta H Delta S Delta G Chart
Mar 25, 2025
-
A Chemical Equation Is Balanced When
Mar 25, 2025
-
What Is The Average Kinetic Energy
Mar 25, 2025
-
What Are The Building Blocks Of Macromolecules
Mar 25, 2025
-
Chemistry The Molecular Nature Of Matter And Change 8th Edition
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Are Chemical Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
