Group 3 In The Periodic Table
Muz Play
Mar 28, 2025 · 7 min read
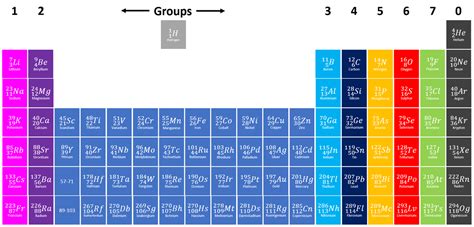
Table of Contents
Group 3: Delving into the Rare Earth Elements and Beyond
Group 3 of the periodic table, often referred to as Scandium Group, presents a fascinating blend of properties and challenges. Unlike other groups, its definition and even the elements included have been subject to debate and revision throughout the history of chemical understanding. This article delves deep into the intricacies of Group 3, exploring its elements, their unique characteristics, applications, and the ongoing scientific discussions surrounding its classification.
Defining Group 3: A Historical Perspective and Ongoing Debate
The classification of Group 3 has been a source of ongoing discussion among chemists. Traditionally, Group 3 consisted only of scandium (Sc), yttrium (Y), and lanthanum (La). However, the inclusion of the lanthanides (elements 57-71) and actinides (elements 89-103) complicates the picture. These f-block elements share similar chemical properties with the elements traditionally assigned to Group 3, leading to varying interpretations of the group's composition.
Some definitions restrict Group 3 to the three elements Sc, Y, and La, emphasizing their similar outer electron configurations and resulting chemical behavior. This simpler definition allows for easier understanding and study of their shared properties. Others include the entire f-block within Group 3, acknowledging the significant chemical similarities and the complexities of electronic structure. This broader definition reflects the complex interplay between the d- and f-orbital electrons.
The debate highlights the limitations of a strictly vertical categorization scheme in the periodic table, particularly for elements exhibiting unique electronic configurations and resulting properties. The ambiguity underscores the ongoing development and refinement of our understanding of the elements and their relationships.
The Elements of Group 3: A Closer Look
Let's delve into the individual elements of Group 3, considering both the traditional and broader definitions:
Scandium (Sc): The Pioneer
Scandium, the lightest element in the group, is a relatively rare transition metal. Its properties sit between those of aluminum and yttrium, showing some similarities to both. It’s a silvery-white metal with high melting and boiling points. While its applications are relatively limited due to its scarcity and high cost, scandium finds niche uses:
- High-intensity lighting: Scandium iodide is used in high-intensity discharge lamps, producing a bright, intense light.
- Aluminum alloys: Small additions of scandium significantly improve the strength and weldability of aluminum alloys, making them suitable for aerospace applications.
- Fuel cells: Emerging applications include its use in fuel cells as a catalyst component.
Yttrium (Y): A Versatile Metal
Yttrium, somewhat more abundant than scandium, is another silvery-white metal with excellent mechanical properties. Its primary applications leverage its unique physical characteristics:
- Phosphors: Yttrium compounds are crucial in the production of color television screens and various other phosphors, contributing to the vibrant colors displayed.
- Superconductors: Certain yttrium compounds exhibit superconductivity at relatively high temperatures, opening up potential applications in advanced electronics.
- Lasers: Yttrium aluminum garnet (YAG) lasers find use in various applications, from medical procedures to industrial material processing.
- Ceramics: Yttrium oxide is used in the production of high-performance ceramics and other materials.
Lanthanum (La): The Bridge to the Lanthanides
Lanthanum is the first element in the lanthanide series and acts as a bridge between the traditional Group 3 elements and the f-block elements. It possesses distinct properties, exhibiting a greater reactivity compared to scandium and yttrium. Some of its key applications include:
- Catalytic converters: Lanthanum oxide is a component in catalytic converters, promoting the efficient conversion of harmful exhaust gases into less harmful substances.
- Nickel-metal hydride batteries: Lanthanum alloys are used in nickel-metal hydride batteries, contributing to their energy storage capacity.
- Optical glass: Lanthanum oxide is added to optical glass to increase its refractive index, enhancing the quality of lenses and optical instruments.
The Lanthanides: A Family of Similar Elements
The lanthanides (cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium) represent a unique group of elements with extremely similar chemical properties. This similarity arises from the gradual filling of the 4f electron shell, shielding the outermost electrons from significant changes in chemical behavior. However, their subtle differences in electronic structure lead to a spectrum of unique properties and applications:
- Magnets: Certain lanthanides, like neodymium and dysprosium, are essential components of powerful permanent magnets used in various applications, including electric motors and wind turbines.
- Catalysis: Several lanthanides, notably cerium, are used as catalysts in various chemical processes.
- Nuclear applications: Some lanthanides, such as promethium (a radioactive element), find use in specialized applications related to nuclear technology.
- Medical applications: Gadolinium compounds are used as contrast agents in magnetic resonance imaging (MRI).
The Actinides: Radioactive and Highly Reactive
The actinides (actinium, thorium, protactinium, uranium, neptunium, plutonium, americium, curium, berkelium, californium, einsteinium, fermium, mendelevium, nobelium, and lawrencium) are all radioactive elements with a similar pattern of electron configuration as the lanthanides. However, their high reactivity and radioactivity present challenges in their handling and applications:
- Nuclear fuel: Uranium and plutonium are crucial in nuclear reactors as fuel sources.
- Nuclear weapons: Plutonium is utilized in the production of nuclear weapons.
- Radioactive tracers: Certain actinides are employed as radioactive tracers in various scientific and medical applications.
- Research: The heavier actinides are mainly studied in research settings due to their rarity and radioactivity.
Applications of Group 3 Elements: A Broad Spectrum
The applications of Group 3 elements span a diverse range of industries and technologies:
- Lighting: Scandium iodide's use in high-intensity lighting exemplifies the group’s contribution to illumination technology.
- Electronics: The lanthanides play a vital role in the development of high-performance electronics, particularly in magnets and memory devices.
- Materials Science: The unique properties of Group 3 elements are crucial in materials science, influencing the development of advanced alloys, ceramics, and composites.
- Medicine: Gadolinium compounds used in MRI highlight the contribution of Group 3 elements to medical imaging and diagnostics.
- Energy: The use of lanthanides in batteries and the role of uranium and plutonium in nuclear energy exemplify their contribution to energy technology.
Extraction and Separation: Challenges and Innovations
Extracting and separating the elements of Group 3 presents significant challenges due to the similar chemical properties of the lanthanides and actinides. Sophisticated techniques are employed, including:
- Ion exchange chromatography: This method separates the elements based on their slight differences in ionic radii and charge densities.
- Solvent extraction: This involves using specific solvents to selectively extract the desired element from a mixture.
- Crystallization: This technique utilizes the differences in solubility of various salts to separate the elements.
Environmental Concerns and Sustainability
The extraction and processing of Group 3 elements can have environmental impacts, particularly regarding the generation of radioactive waste (in the case of actinides) and the disposal of hazardous byproducts. Sustainable practices and responsible waste management are crucial to minimizing these negative effects.
Future Research and Development
Research in Group 3 elements is ongoing, driven by the need for new materials with improved properties and the development of more efficient and sustainable extraction techniques. Areas of focus include:
- Novel applications of lanthanides: Exploring the unique properties of individual lanthanides for applications in advanced technologies, such as quantum computing and next-generation energy storage.
- Sustainable extraction methods: Developing more environmentally friendly ways to extract and process these elements, reducing waste and minimizing environmental impact.
- Understanding actinide chemistry: Further research into the chemistry of actinides is crucial for improving nuclear waste management and developing new applications for these radioactive elements.
Conclusion: A Group of Significance
Group 3 of the periodic table, despite its complex definition and the challenges in handling its elements, plays a vital role in modern technology and scientific advancement. The unique properties of its elements, from the relatively common scandium and yttrium to the strategically important lanthanides and the radioactive actinides, continue to drive innovation across diverse sectors. Further research and development in this field are crucial not only for unlocking the full potential of these elements but also for ensuring responsible and sustainable use to benefit humanity while mitigating environmental impacts. The ongoing debates surrounding the definition of Group 3 itself underscores the dynamic nature of our understanding of the periodic table and its continuing evolution as scientific knowledge expands.
Latest Posts
Latest Posts
-
What Is The Electron Configuration Of Beryllium
Mar 31, 2025
-
When Do You Consider Log Diterminants Similar
Mar 31, 2025
-
Find The Rectangular Equation And Eliminate The Parameters
Mar 31, 2025
-
How To Calculate The Enthalpy Of Fusion
Mar 31, 2025
-
How Does Meiosis Generate Genetic Diversity
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Group 3 In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
