Has A Definite Shape And Volume
Muz Play
Mar 23, 2025 · 6 min read
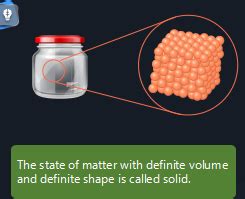
Table of Contents
Has a Definite Shape and Volume: Exploring the World of Solids
The simple statement, "has a definite shape and volume," immediately points us towards a fundamental concept in science: the solid state of matter. Unlike liquids and gases, which conform to the shape of their containers and readily compress, solids possess a unique and defining characteristic: they maintain a fixed shape and volume regardless of their environment (barring extreme pressure or temperature). This seemingly straightforward property unlocks a vast array of fascinating physical and chemical behaviours, influencing everything from the construction of skyscrapers to the intricacies of biological molecules.
Understanding the Microscopic Structure of Solids
The key to understanding why solids have a definite shape and volume lies at the atomic and molecular level. The particles within a solid – atoms, ions, or molecules – are held together by strong intermolecular forces. These forces, ranging from covalent bonds (shared electrons) to ionic bonds (electrostatic attraction) and metallic bonds (sea of delocalized electrons), restrict the movement of the constituent particles.
Crystalline Solids: Order and Regularity
Many solids exhibit a highly ordered arrangement of their constituent particles. These are known as crystalline solids. Think of a meticulously arranged stack of oranges – each orange represents an atom or molecule, occupying a specific position within a repeating three-dimensional pattern called a crystal lattice. The regularity and strength of the bonds within this lattice give crystalline solids their characteristic rigidity and definite shape. Examples include table salt (sodium chloride), diamonds (carbon), and quartz (silicon dioxide).
The type of crystal lattice influences the properties of the solid. For instance, the cubic crystal structure of diamond contributes to its exceptional hardness, while the layered structure of graphite allows for easy exfoliation and use in pencils. The study of crystallography is dedicated to understanding these structures and their implications.
Amorphous Solids: Disorder and Flexibility
Not all solids are crystalline. Amorphous solids, also known as non-crystalline solids, lack the long-range order found in crystalline materials. Their constituent particles are arranged randomly, like a jumbled pile of oranges. This lack of order means their properties are often less uniform and more susceptible to variations in temperature and pressure.
Amorphous solids generally exhibit greater flexibility than crystalline solids. Glass, rubber, and plastics are prime examples of amorphous solids. The disordered structure of glass allows it to be easily molded when heated, while the flexibility of rubber stems from the ability of its polymer chains to move and rearrange. However, even amorphous solids maintain a definite volume at a given temperature and pressure.
The Role of Intermolecular Forces
The strength of intermolecular forces is directly related to a solid's ability to maintain its shape and volume. Stronger forces lead to more rigid solids with higher melting points, whereas weaker forces result in solids that are more easily deformed or have lower melting points.
Covalent Bonds: The Strongest Link
Covalent bonds represent the strongest type of intermolecular force. Atoms share electrons to achieve a stable electron configuration, forming strong, directional bonds. This results in incredibly strong and rigid solids, as seen in diamond and quartz. The high melting points of these materials reflect the substantial energy required to break these bonds.
Ionic Bonds: Electrostatic Attraction
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. One atom donates an electron to another, creating a positively charged cation and a negatively charged anion. These ions are then held together by strong coulombic forces, resulting in crystalline solids with relatively high melting points, such as sodium chloride (table salt).
Metallic Bonds: A Sea of Electrons
Metallic bonds are a unique type of bonding found in metals. Valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged metal ions. This allows for excellent electrical and thermal conductivity, as well as malleability and ductility. The relatively strong metallic bonds contribute to the definite shape and volume of metallic solids.
Van der Waals Forces: Weaker Interactions
Van der Waals forces are weaker intermolecular forces that arise from temporary fluctuations in electron distribution around atoms or molecules. These forces are responsible for the cohesion in many molecular solids, such as ice and dry ice. Although weaker than covalent, ionic, or metallic bonds, Van der Waals forces still contribute to the maintenance of shape and volume in these solids, albeit often to a lesser extent.
Factors Influencing Shape and Volume
While the inherent properties of a solid dictate its ability to maintain shape and volume, external factors can also play a role.
Temperature: The Energy Factor
Increasing temperature increases the kinetic energy of the particles within a solid. This increased energy can overcome the intermolecular forces holding the particles together, causing the solid to melt and lose its definite shape. The melting point is a crucial property, indicating the temperature at which a solid transitions into a liquid.
Pressure: External Force
Applying external pressure can compress a solid, slightly reducing its volume. However, the change in volume is typically small compared to liquids and gases because the strong intermolecular forces prevent significant particle rearrangement. Very high pressures can, however, induce structural changes, potentially leading to phase transitions.
The Importance of Solids in Our World
The unique property of solids – their ability to maintain a definite shape and volume – underpins countless aspects of our daily lives. The construction industry relies heavily on the strength and stability of solid materials like concrete, steel, and wood. Electronic devices rely on the semiconducting properties of crystalline silicon. Biological systems depend on the structural integrity of proteins and other biomolecules, which are essentially highly organized solids.
Without the inherent stability of solids, our world would be drastically different, a chaotic mix of flowing liquids and expanding gases. The very existence of our built environment, and indeed, our bodies, hinges on the fundamental properties of solids, their ability to maintain a fixed shape and volume, allowing us to construct and interact with the world around us.
Conclusion: A Solid Foundation for Understanding Matter
The simple statement, "has a definite shape and volume," encapsulates a deep understanding of the solid state of matter. This property, stemming from the strong intermolecular forces that bind the constituent particles, dictates the physical and chemical characteristics of solids, influencing their applications across a vast range of disciplines. From the towering skyscrapers that define our skylines to the microscopic structures that govern life itself, the world as we know it is built upon the solid foundation of materials that maintain a definite shape and volume. Continued research into the intricacies of solid-state physics and chemistry promises to unveil even more about the fascinating behaviors of these fundamental building blocks of our world, leading to the development of new materials and technologies with revolutionary applications. The seemingly simple concept of a definite shape and volume is thus a cornerstone of scientific understanding, unlocking a universe of possibilities in engineering, materials science, and beyond.
Latest Posts
Latest Posts
-
Unit Of Permeability Of Free Space
Mar 24, 2025
-
Do Prokaryotes And Eukaryotes Have Ribosomes
Mar 24, 2025
-
Time Magazine 100 Most Influential 20th Century
Mar 24, 2025
-
Where Are The Neutrons Located In The Atom
Mar 24, 2025
-
Acids Release Which Type Of Ion In Water
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Has A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
