Acids Release Which Type Of Ion In Water
Muz Play
Mar 24, 2025 · 6 min read
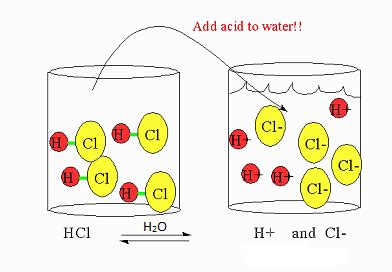
Table of Contents
Acids: Understanding the Release of Hydrogen Ions in Water
Acids are ubiquitous in our daily lives, from the citric acid in oranges to the sulfuric acid used in car batteries. Understanding their behavior, particularly their interaction with water and the subsequent release of ions, is crucial in various fields, including chemistry, biology, and environmental science. This article delves deep into the nature of acids and explores the specific type of ion they release when dissolved in water: the hydrogen ion (H⁺). We will examine the different strengths of acids, the concept of pH, and the implications of acid-base reactions.
What are Acids?
Acids are chemical substances that donate protons (H⁺ ions) to other substances, a characteristic known as proton donation. This definition, proposed by Brønsted and Lowry, is one of the most widely accepted ways to define acids. Another definition, proposed by Arrhenius, defines acids as substances that increase the concentration of hydrogen ions (H⁺) when dissolved in water. While the Arrhenius definition is simpler, the Brønsted-Lowry definition is more comprehensive, encompassing reactions that don't necessarily involve water.
Regardless of the definition used, the core characteristic remains: acids increase the concentration of hydrogen ions (H⁺) in a solution. This is the key to understanding their behavior in aqueous solutions.
The Behavior of Acids in Water: Dissociation and Ionization
When an acid is dissolved in water, it undergoes dissociation or ionization. This process involves the breaking apart of the acid molecule into its constituent ions. For example, consider hydrochloric acid (HCl), a strong acid. In water, it almost completely dissociates into hydrogen ions (H⁺) and chloride ions (Cl⁻):
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The (aq) notation indicates that the species are dissolved in water. This dissociation is essentially complete for strong acids, meaning a high percentage of the acid molecules break apart into ions.
However, weak acids only partially dissociate. Acetic acid (CH₃COOH), found in vinegar, is a classic example. Its dissociation is represented as:
CH₃COOH(aq) ⇌ H⁺(aq) + CH₃COO⁻(aq)
The double arrow (⇌) signifies an equilibrium reaction. This means that the dissociation is reversible, with some undissociated acetic acid molecules coexisting with hydrogen ions and acetate ions (CH₃COO⁻). The extent of dissociation is quantified by the acid dissociation constant (Ka), a measure of the acid's strength.
Strong Acids vs. Weak Acids: A Key Distinction
The extent of dissociation determines whether an acid is classified as strong or weak.
Strong acids completely or almost completely dissociate in water, releasing a high concentration of hydrogen ions. Examples include:
- Hydrochloric acid (HCl)
- Sulfuric acid (H₂SO₄)
- Nitric acid (HNO₃)
- Perchloric acid (HClO₄)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
Weak acids, on the other hand, only partially dissociate, resulting in a low concentration of hydrogen ions. Examples include:
- Acetic acid (CH₃COOH)
- Carbonic acid (H₂CO₃)
- Phosphoric acid (H₃PO₄)
- Hydrofluoric acid (HF)
- Benzoic acid (C₇H₆O₂)
The difference in dissociation behavior has significant implications for their reactivity and properties. Strong acids are highly reactive and corrosive, while weak acids are generally less reactive.
The Role of Hydrogen Ions (H⁺) in Aqueous Solutions
The release of hydrogen ions (H⁺) is the defining characteristic of acids. These ions are responsible for the acidic properties of the solution. However, it's important to understand that free H⁺ ions do not exist independently in water. Instead, they readily react with water molecules to form hydronium ions (H₃O⁺):
H⁺(aq) + H₂O(l) → H₃O⁺(aq)
The hydronium ion is a more accurate representation of the protonated water molecule. While we often represent the acidic reaction using H⁺ for simplicity, it's crucial to remember the actual species in solution is H₃O⁺.
pH: A Measure of Acidity
The concentration of hydrogen ions (or hydronium ions) in a solution is expressed using the pH scale. The pH scale is logarithmic, meaning a change of one pH unit represents a tenfold change in hydrogen ion concentration. A pH of 7 is considered neutral, representing pure water. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are basic (alkaline).
The lower the pH value, the higher the concentration of hydrogen ions and the stronger the acid. Strong acids typically have pH values close to 0, while weak acids have pH values ranging from slightly below 7 to closer to 7.
The Importance of Understanding Acid Dissociation
Understanding the release of hydrogen ions by acids is crucial in various contexts:
- Chemistry: Predicting the outcome of chemical reactions involving acids.
- Biology: Understanding the role of acids in biological processes, such as digestion and maintaining blood pH.
- Environmental science: Studying the effects of acid rain on ecosystems and the environment.
- Industrial processes: Controlling and manipulating the acidity of solutions in various industrial applications.
- Medicine: Understanding the impact of acids on the human body and the treatment of acid-related conditions.
Examples of Acidic Reactions and their Importance:
- Digestion: Hydrochloric acid (HCl) in the stomach plays a crucial role in breaking down food. The low pH of the stomach acid helps activate digestive enzymes and kill harmful bacteria.
- Acid Rain: The burning of fossil fuels releases sulfur dioxide and nitrogen oxides into the atmosphere, which react with water to form sulfuric acid and nitric acid. This acid rain can damage ecosystems, buildings, and infrastructure.
- Battery Chemistry: Sulfuric acid is a key component in lead-acid batteries, providing the necessary electrolyte for the electrochemical reactions that produce electricity.
- Food Preservation: Acids such as acetic acid (vinegar) and citric acid (lemon juice) are used as preservatives in food due to their antimicrobial properties.
Beyond the Basics: Polyprotic Acids and Acid Strength
The discussion so far has largely focused on monoprotic acids, which donate only one proton per molecule. However, some acids, known as polyprotic acids, can donate more than one proton. Sulfuric acid (H₂SO₄), for example, is a diprotic acid, donating two protons in successive steps:
H₂SO₄(aq) → H⁺(aq) + HSO₄⁻(aq)
HSO₄⁻(aq) ⇌ H⁺(aq) + SO₄²⁻(aq)
The second dissociation is an equilibrium process, unlike the first one. The different dissociation constants for each step indicate different strengths for each proton donation.
Understanding the stepwise dissociation of polyprotic acids is essential for accurately predicting the behaviour of these acids in solution.
The strength of an acid, as mentioned earlier, is determined by its ability to donate protons. Strong acids completely dissociate, releasing all their protons while weak acids do this only partially. This difference significantly impacts their reactivity and their use in various applications.
Conclusion: The Central Role of Hydrogen Ions in Acidic Behavior
The release of hydrogen ions (H⁺), or more accurately, the formation of hydronium ions (H₃O⁺), is the fundamental characteristic of acids. This process underlies the acidic properties of solutions and has significant implications across various fields. Understanding the strength of acids, the concept of pH, and the behavior of polyprotic acids is vital for appreciating the diverse roles of acids in chemistry, biology, and the environment. The continuous exploration and deeper understanding of acid dissociation are crucial to advancing knowledge and innovation in countless applications. From the development of new materials to environmental protection, the knowledge of acid behavior remains at the forefront of scientific and technological advancements.
Latest Posts
Latest Posts
-
Does A Gas Have A Definite Shape And Volume
Mar 28, 2025
-
Limit Of Function Of Two Variables
Mar 28, 2025
-
What Controls What Enters And Leaves A Cell
Mar 28, 2025
-
Time Rate Of Change Of Momentum
Mar 28, 2025
-
Definition Of Marketing By Philip Kotler
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Acids Release Which Type Of Ion In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
