How Are Electrons Arranged Around The Nucleus Of An Atom
Muz Play
Apr 01, 2025 · 5 min read
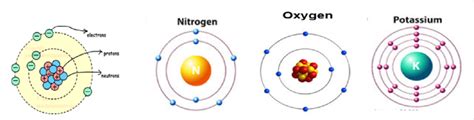
Table of Contents
How Are Electrons Arranged Around the Nucleus of an Atom?
Understanding the arrangement of electrons around an atom's nucleus is fundamental to comprehending chemistry and the properties of matter. This seemingly simple question unlocks a complex world of quantum mechanics, shaping chemical bonds, reactivity, and the very nature of the elements. This article will delve deep into this fascinating topic, exploring the historical development of electron configuration models, the principles governing electron placement, and the implications of this arrangement for chemical behavior.
Early Models and the Limitations of Classical Physics
Before diving into the accepted model, it's essential to acknowledge the historical context. Early models, influenced by classical physics, failed to accurately depict electron behavior. The plum pudding model, for instance, proposed electrons embedded within a positively charged sphere, like plums in a pudding. This model, however, couldn't explain the observed stability of atoms or the discrete nature of spectral lines.
Rutherford's nuclear model, while revolutionary in proposing a dense, positively charged nucleus surrounded by orbiting electrons, still faced significant challenges. Classical electromagnetism predicted that orbiting electrons, constantly accelerating, would emit electromagnetic radiation, lose energy, and spiral into the nucleus, causing atoms to collapse. This clearly contradicted the observed stability of matter.
The Quantum Revolution: Introducing Orbitals and Quantum Numbers
The resolution came with the advent of quantum mechanics. This revolutionary theory replaced the classical view of electrons as tiny particles orbiting the nucleus with a probabilistic description, suggesting electrons exist in regions of space called orbitals. These orbitals aren't simply paths, but rather regions of high probability of finding an electron.
The location and energy of an electron within an atom are described by four quantum numbers:
1. Principal Quantum Number (n)
This number defines the electron shell or energy level. Higher values of n indicate higher energy levels and greater distance from the nucleus. n can be any positive integer (1, 2, 3, and so on). Shells are often labeled with letters (K, L, M, N, etc.), corresponding to n = 1, 2, 3, 4, respectively.
2. Azimuthal Quantum Number (l)
This number specifies the subshell or orbital type within a shell. For a given n, l can take on integer values from 0 to n - 1. Subshells are designated by letters:
- l = 0: s subshell (spherical orbital)
- l = 1: p subshell (dumbbell-shaped orbitals)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Each subshell can hold a specific number of electrons: s (2), p (6), d (10), and f (14).
3. Magnetic Quantum Number (ml)
This number defines the orbital orientation within a subshell. For a given l, ml can take on integer values from -l to +l, including 0. For example, a p subshell (l=1) has three orbitals (ml = -1, 0, +1), oriented along the x, y, and z axes.
4. Spin Quantum Number (ms)
This number describes the intrinsic angular momentum of an electron, often visualized as spin. Each electron can have a spin of +1/2 (spin up) or -1/2 (spin down). This is crucial because of the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons, with opposite spins.
Filling Orbitals: The Aufbau Principle and Hund's Rule
The arrangement of electrons in an atom follows specific rules:
The Aufbau Principle
This principle states that electrons fill the lowest energy levels first. The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... However, exceptions exist due to subtle energy level variations.
Hund's Rule
This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration. Each electron in a subshell will have the same spin before pairing begins.
Electron Configurations and their Chemical Implications
The electron configuration of an atom is a shorthand notation showing the arrangement of electrons in its orbitals. For example, the electron configuration of oxygen (atomic number 8) is 1s²2s²2p⁴. This means it has two electrons in the 1s orbital, two in the 2s orbital, and four in the 2p orbitals.
The outermost electrons, known as valence electrons, are crucial for determining an element's chemical reactivity. Elements in the same group (vertical column) of the periodic table have the same number of valence electrons and often exhibit similar chemical behavior. For example, the alkali metals (Group 1) all have one valence electron, making them highly reactive.
The arrangement of electrons also influences other properties like ionization energy, electronegativity, and atomic radius. Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration, often resembling that of a noble gas (full outermost shell). This drive towards stability underpins the formation of chemical bonds.
Beyond the Basics: Exceptions and Refinements
While the Aufbau principle provides a good general guideline, exceptions exist. For example, chromium (Cr) and copper (Cu) have unusual electron configurations due to the relatively close energy levels of the 3d and 4s orbitals. These exceptions highlight the complexity of electron-electron interactions and the limitations of simple models in accurately predicting electron configuration in all cases.
Furthermore, the orbital model simplifies the reality of electron behavior. Electrons don't simply occupy specific orbitals; their behavior is governed by probability distributions. Advanced quantum mechanical calculations are needed to obtain a more precise understanding of electron density and distribution within an atom.
Conclusion: A Journey into the Quantum World
The arrangement of electrons around the nucleus is not a simple, easily visualized concept. It's a testament to the power and complexity of quantum mechanics. Understanding this arrangement is paramount to understanding the periodic table, chemical bonding, and the diverse properties of matter. While simplified models provide a useful framework, the true picture involves probabilities, subtle energy differences, and complex electron-electron interactions. Further exploration into quantum chemistry and advanced computational methods reveals the intricacies and beauty of this fundamental aspect of atomic structure. Continuing research continually refines our understanding of this fascinating quantum world, constantly pushing the boundaries of our knowledge.
Latest Posts
Latest Posts
-
In Which Phase Of Cellular Respiration Is Water Made
Apr 02, 2025
-
How To Measure Current On A Breadboard
Apr 02, 2025
-
Chapter 1 Lab Investigation The Language Of Anatomy
Apr 02, 2025
-
How Is Chemistry Used In Forensic Science
Apr 02, 2025
-
How Do You Square A Radical
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Are Electrons Arranged Around The Nucleus Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
