How Are Temperature And Kinetic Energy Related
Muz Play
Mar 27, 2025 · 6 min read
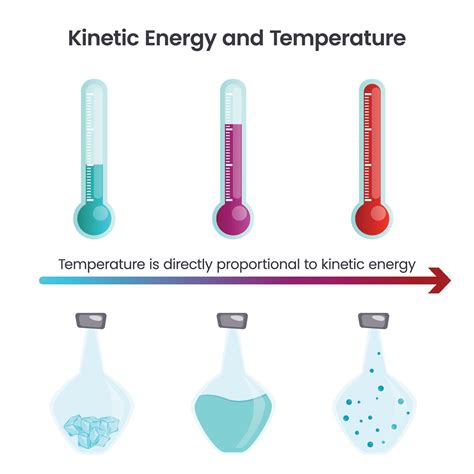
Table of Contents
How Are Temperature and Kinetic Energy Related? A Deep Dive
Understanding the relationship between temperature and kinetic energy is fundamental to grasping many concepts in physics and chemistry. It's a cornerstone of thermodynamics and explains phenomena ranging from the boiling of water to the behavior of gases in balloons. This article will explore this relationship in detail, starting with the basics and delving into more complex aspects.
The Fundamental Connection: Temperature as Average Kinetic Energy
At its core, temperature is a measure of the average kinetic energy of the particles (atoms or molecules) within a substance. Kinetic energy, denoted as KE, is the energy an object possesses due to its motion. The formula for kinetic energy is:
KE = 1/2 * mv²
Where:
- m is the mass of the particle
- v is the velocity (speed and direction) of the particle
This means that particles with higher velocities have greater kinetic energy. Temperature, therefore, reflects the average speed of these particles. A higher temperature indicates that the particles are, on average, moving faster and thus possess higher average kinetic energy. Conversely, a lower temperature implies slower particle motion and lower average kinetic energy.
Microscopic vs. Macroscopic View
It's crucial to differentiate between the microscopic perspective (individual particle behavior) and the macroscopic perspective (observable properties of the substance). While individual particles within a substance are constantly colliding and changing speed, temperature represents the average kinetic energy across all the particles. This averaging process smooths out the fluctuations in individual particle energies, providing a meaningful macroscopic measure.
The Role of Molecular Motion
The type of motion influencing kinetic energy and thus temperature depends on the state of matter:
- Solids: Particles in solids are tightly packed and vibrate in place. Their kinetic energy is largely due to these vibrational movements. Increasing temperature increases the amplitude (intensity) of these vibrations.
- Liquids: Particles in liquids have more freedom of movement than in solids. They exhibit translational (movement from place to place), rotational, and vibrational motion. Increased temperature leads to more vigorous motion in all these forms.
- Gases: Particles in gases are widely dispersed and move freely. Their kinetic energy is primarily due to translational motion, constantly colliding with each other and the container walls. Higher temperatures mean faster and more frequent collisions.
Beyond the Average: Distribution of Kinetic Energies
While temperature represents the average kinetic energy, it's essential to remember that not all particles within a substance have the same kinetic energy. Their energies follow a statistical distribution, often described by the Maxwell-Boltzmann distribution. This distribution shows that at any given temperature, a range of kinetic energies exists among the particles, with a peak representing the most probable kinetic energy.
Impact of Temperature on the Distribution
Increasing the temperature shifts the Maxwell-Boltzmann distribution to higher energies. The peak of the distribution moves to higher kinetic energies, indicating that the average kinetic energy has increased. The distribution also broadens, suggesting a wider range of kinetic energies among the particles.
Implications for Phase Transitions
This distribution of kinetic energies is critical in understanding phase transitions. For example, for a liquid to boil and transition to a gas, a significant fraction of its particles needs to possess enough kinetic energy to overcome the intermolecular forces holding them together. Increasing the temperature increases the proportion of particles with sufficient energy to escape the liquid phase, leading to boiling.
Temperature Scales and Kinetic Energy
Different temperature scales (Celsius, Fahrenheit, Kelvin) are simply different ways of expressing the same underlying concept: the average kinetic energy of particles. However, the Kelvin scale is unique because its zero point (0 K, or absolute zero) corresponds to the absence of all thermal motion. At absolute zero, the average kinetic energy of particles theoretically reaches zero. It's important to note that it's impossible to reach absolute zero in practice due to quantum mechanical effects.
Specific Heat Capacity and Kinetic Energy
Specific heat capacity is the amount of energy required to raise the temperature of one unit of mass of a substance by one degree. This property is directly related to how effectively a substance can store kinetic energy. Substances with high specific heat capacities require more energy to increase their average kinetic energy (and temperature) than those with low specific heat capacities. This difference stems from the complexity of the interactions between particles and the amount of energy needed to overcome these interactions and increase particle motion.
Temperature and Kinetic Energy in Different Systems
The relationship between temperature and kinetic energy applies to a wide range of systems, including:
- Ideal Gases: The kinetic theory of gases provides a precise mathematical relationship between temperature, pressure, volume, and the average kinetic energy of gas molecules.
- Solids: The vibrational motion of atoms in a solid lattice directly reflects its temperature. Increased temperature leads to increased vibrational amplitudes and ultimately to thermal expansion.
- Plasmas: In plasmas, which are highly ionized gases, the kinetic energy of both ions and electrons contributes to the temperature.
- Stars: The incredibly high temperatures in stars are due to the immense kinetic energy of their constituent particles, primarily hydrogen nuclei undergoing nuclear fusion.
Applications and Real-World Examples
The relationship between temperature and kinetic energy has far-reaching implications and applications across various fields:
- Engine Design: Understanding how temperature affects the kinetic energy of fuel molecules is crucial for optimizing engine efficiency and performance.
- Material Science: The properties of materials are heavily influenced by the kinetic energy of their constituent particles. High temperatures can cause material degradation, while controlled heating can be used for processes like annealing and tempering.
- Meteorology: Atmospheric temperature is a key factor influencing weather patterns, as it affects the kinetic energy of air molecules and thus wind speeds and precipitation.
- Chemical Reactions: The rate of chemical reactions is highly dependent on the kinetic energy of the reactant molecules. Higher temperatures increase the likelihood of successful collisions leading to product formation.
Conclusion: A Dynamic Relationship
The relationship between temperature and kinetic energy is fundamental to our understanding of the physical world. While temperature provides a macroscopic measure of the average kinetic energy of particles, the underlying microscopic behavior of individual particles, their distribution of kinetic energies, and the nature of their interactions paint a more complete picture of this dynamic relationship. Understanding this connection is key to unlocking insights in numerous scientific disciplines and technological advancements. From the design of efficient engines to the prediction of weather patterns, the implications of this relationship are vast and ever-expanding. Further exploration into the complexities of this relationship continues to be a fertile ground for scientific discovery and technological innovation.
Latest Posts
Latest Posts
-
How Does Fermentation Allow Glycolysis To Continue
Mar 30, 2025
-
Which Subatomic Particle Carries A Positive Charge
Mar 30, 2025
-
Signs A Chemical Reaction Has Occurred
Mar 30, 2025
-
Duties Of An Agent In Law
Mar 30, 2025
-
What Is The Symbol For Population Variance
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Are Temperature And Kinetic Energy Related . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
