How Can A Molecule With Polar Bonds Be Nonpolar
Muz Play
Mar 16, 2025 · 6 min read
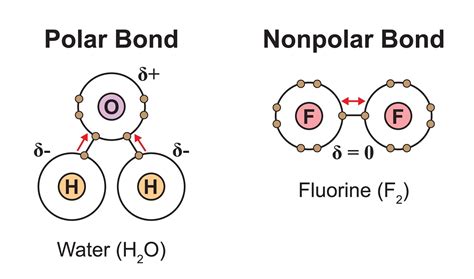
Table of Contents
How Can a Molecule with Polar Bonds Be Nonpolar?
Understanding molecular polarity is crucial in chemistry, as it dictates a molecule's properties and behavior. A molecule's polarity is determined by the presence of polar bonds and the molecule's overall geometry. This article delves deep into the fascinating concept of how a molecule possessing polar bonds can still exhibit nonpolar characteristics. We'll explore the underlying principles, providing examples and clarifying the nuances involved.
Understanding Polar Bonds and Molecular Polarity
Before diving into the central question, let's establish a firm understanding of the fundamental concepts.
Polar Bonds: The Unequal Sharing of Electrons
A polar bond arises when two atoms with significantly different electronegativities bond covalently. Electronegativity is an atom's ability to attract electrons towards itself within a chemical bond. The more electronegative atom pulls the shared electrons closer, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This charge separation creates an electric dipole moment. The greater the difference in electronegativity, the more polar the bond. Examples of highly polar bonds include those between hydrogen and oxygen (O-H) or hydrogen and chlorine (H-Cl).
Molecular Polarity: The Sum of Individual Bond Dipoles
Molecular polarity, unlike bond polarity, considers the overall distribution of charge within an entire molecule. It's the vector sum of all the individual bond dipoles. Even if a molecule contains polar bonds, the molecule itself might be nonpolar if these bond dipoles cancel each other out due to the molecule's symmetry. This cancellation results in a zero net dipole moment.
The Role of Molecular Geometry
The spatial arrangement of atoms within a molecule, its geometry, plays a pivotal role in determining its overall polarity. The following molecular geometries are crucial in determining if a molecule with polar bonds is nonpolar.
Linear Geometry
A linear molecule has all atoms arranged in a straight line (bond angle of 180°). If the molecule consists of only two atoms with different electronegativities, it will always be polar. However, a linear molecule with three or more atoms might be nonpolar if the individual bond dipoles are equal in magnitude and opposite in direction, causing them to cancel out. A classic example is carbon dioxide (CO₂). Each C=O bond is polar, but the symmetrical linear arrangement means the bond dipoles cancel, resulting in a nonpolar molecule.
Trigonal Planar Geometry
Molecules with trigonal planar geometry have a central atom surrounded by three atoms in a flat triangular arrangement (bond angle of approximately 120°). Similar to linear molecules, symmetrical trigonal planar molecules with identical surrounding atoms can be nonpolar. Boron trifluoride (BF₃) is a prime example. Each B-F bond is polar, yet the symmetrical arrangement ensures the bond dipoles cancel, resulting in a nonpolar molecule.
Tetrahedral Geometry
Tetrahedral geometry features a central atom bonded to four atoms located at the corners of a tetrahedron (bond angle of approximately 109.5°). A molecule with tetrahedral geometry can be nonpolar if all four surrounding atoms are identical. Methane (CH₄) exemplifies this: although each C-H bond is slightly polar, the symmetrical arrangement perfectly cancels out the bond dipoles. However, a substitution of even one hydrogen atom with a different atom will break the symmetry, making the molecule polar. For example, Chloromethane (CH₃Cl) is polar.
Other Geometries
Other geometries, such as trigonal bipyramidal and octahedral, also exhibit similar principles. Symmetrical arrangements of identical surrounding atoms lead to nonpolar molecules despite the presence of polar bonds. The crucial element is the symmetrical distribution of charge, which cancels out any individual bond dipole moments.
Examples of Molecules with Polar Bonds but Nonpolar Overall
Let's look at several specific examples to solidify our understanding.
1. Carbon Dioxide (CO₂): As mentioned earlier, CO₂ is a linear molecule with two highly polar C=O bonds. However, the symmetry of the molecule ensures the bond dipoles cancel perfectly, leading to a nonpolar overall molecule.
2. Boron Trifluoride (BF₃): BF₃ is a trigonal planar molecule with three polar B-F bonds. The symmetrical arrangement results in the complete cancellation of bond dipoles, yielding a nonpolar molecule.
3. Carbon Tetrachloride (CCl₄): CCl₄ is a tetrahedral molecule with four polar C-Cl bonds. The tetrahedral symmetry results in the cancellation of bond dipoles and a nonpolar molecule.
4. Sulfur hexafluoride (SF₆): SF₆ possesses six polar S-F bonds. Its octahedral geometry ensures that these dipole moments cancel each other completely, rendering the molecule non-polar.
Distinguishing Between Polar and Nonpolar Molecules: Experimental Techniques
Several experimental techniques can determine a molecule's polarity:
-
Measurement of Dipole Moment: The most direct method involves measuring the molecule's dipole moment using techniques like dielectric constant measurements. A nonpolar molecule will exhibit a dipole moment close to zero.
-
Solubility: Polar molecules tend to dissolve in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents (like hexane). This difference in solubility can help distinguish between polar and nonpolar substances.
-
Boiling Point: Polar molecules generally have higher boiling points compared to nonpolar molecules of similar molar mass due to stronger intermolecular forces (dipole-dipole interactions).
-
Spectroscopic Techniques: Techniques like infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy can provide indirect evidence of molecular polarity based on the observed spectra.
Consequences of Molecular Polarity: Applications in Various Fields
The polarity of a molecule has profound implications across various fields. It influences:
-
Solubility and Miscibility: Polar and nonpolar molecules exhibit different solubility behaviors, impacting their applications in various industries. This understanding is crucial in designing pharmaceuticals, where solubility in bodily fluids is essential.
-
Intermolecular Forces: The presence or absence of a dipole moment significantly impacts intermolecular forces, which in turn affect the physical properties like melting point and boiling point.
-
Chemical Reactivity: The charge distribution within a molecule greatly impacts its reactivity. Polar molecules participate in reactions differently compared to nonpolar molecules.
-
Biological Interactions: Polarity is vital in biological systems. The polarity of water, for example, is crucial for its role as a universal solvent and its involvement in countless biological processes.
Conclusion
Understanding how a molecule with polar bonds can be nonpolar is essential in chemistry. This fascinating phenomenon highlights the interplay between bond polarity and molecular geometry. The symmetry of the molecule dictates the overall polarity. The cancellation of individual bond dipoles due to symmetry leads to a nonpolar molecule despite the presence of individual polar bonds. Recognizing the importance of molecular geometry and its consequences helps us predict and understand the properties of a vast array of chemical compounds and their applications in diverse scientific and technological fields. By understanding these principles, chemists and scientists can accurately predict and manipulate the properties of molecules for various applications.
Latest Posts
Latest Posts
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Can A Molecule With Polar Bonds Be Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
