How Can You Identify A Redox Reaction
Muz Play
Mar 18, 2025 · 6 min read
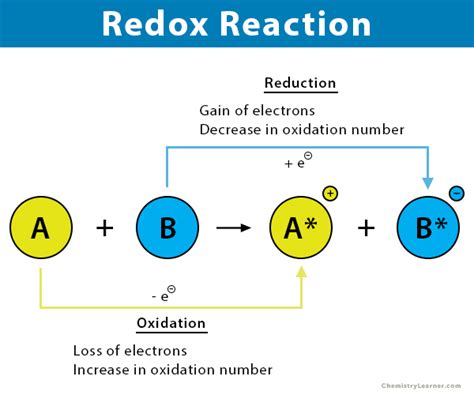
Table of Contents
How Can You Identify a Redox Reaction?
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that underpin a vast array of natural phenomena and industrial applications. From respiration in living organisms to the corrosion of metals and the operation of batteries, redox reactions are everywhere. Understanding how to identify these reactions is crucial for anyone studying chemistry, and this comprehensive guide will equip you with the knowledge and tools to do just that.
Understanding the Fundamentals: Oxidation and Reduction
Before diving into identification techniques, let's solidify our understanding of the core concepts: oxidation and reduction. These processes are always coupled; one cannot occur without the other.
Oxidation: Loss of Electrons
Oxidation is defined as the loss of electrons by an atom, ion, or molecule. This loss results in an increase in the oxidation state (oxidation number) of the species involved. Remember the mnemonic OIL RIG – Oxidation Is Loss, Reduction Is Gain (of electrons).
Examples:
- Na → Na⁺ + e⁻: Sodium (Na) loses one electron to become a sodium ion (Na⁺). This is an oxidation reaction.
- Fe²⁺ → Fe³⁺ + e⁻: Iron(II) ion (Fe²⁺) loses one electron to become an iron(III) ion (Fe³⁺). This is also an oxidation reaction.
Reduction: Gain of Electrons
Reduction, conversely, is the gain of electrons by an atom, ion, or molecule. This gain results in a decrease in the oxidation state of the species involved.
Examples:
- Cl + e⁻ → Cl⁻: A chlorine atom (Cl) gains one electron to become a chloride ion (Cl⁻). This is a reduction reaction.
- Cu²⁺ + 2e⁻ → Cu: A copper(II) ion (Cu²⁺) gains two electrons to become a copper atom (Cu). This is a reduction reaction.
Key Methods for Identifying Redox Reactions
Now that we understand the basics, let's explore the practical methods for identifying redox reactions within chemical equations.
1. Identifying Changes in Oxidation States
This is the most fundamental and reliable method. By carefully assigning oxidation states to all atoms in the reactants and products, we can definitively determine if a redox reaction has occurred.
Steps to Assign Oxidation States:
- Free elements: The oxidation state of an atom in its elemental form is always 0. (e.g., Na, O₂, Cl₂)
- Monatomic ions: The oxidation state of a monatomic ion is equal to its charge. (e.g., Na⁺ = +1, Cl⁻ = -1)
- Oxygen: Oxygen usually has an oxidation state of -2, except in peroxides (e.g., H₂O₂) where it's -1 and in compounds with fluorine where it's positive.
- Hydrogen: Hydrogen usually has an oxidation state of +1, except in metal hydrides (e.g., NaH) where it's -1.
- Group 1 elements: Always +1
- Group 2 elements: Always +2
- The sum of oxidation states in a neutral molecule must equal zero.
- The sum of oxidation states in a polyatomic ion must equal the charge of the ion.
Example: Let's analyze the reaction: 2FeCl₂ + Cl₂ → 2FeCl₃
-
Reactants:
- Fe in FeCl₂: +2 (Cl is -1, and 2Fe + 2Cl = 0, so Fe = +2)
- Cl in FeCl₂: -1
- Cl in Cl₂: 0
-
Products:
- Fe in FeCl₃: +3 (Cl is -1, and Fe + 3Cl = 0, so Fe = +3)
- Cl in FeCl₃: -1
Notice that the oxidation state of iron (Fe) increased from +2 to +3 (oxidation), while the oxidation state of chlorine (Cl) decreased from 0 to -1 (reduction). Since both oxidation and reduction occurred, this is a redox reaction.
2. Identifying the Transfer of Electrons
While calculating oxidation states is precise, sometimes visually observing the electron transfer can be quicker. This method is particularly useful for simpler reactions. Look for changes in ionic charges or the addition/removal of electrons explicitly shown in the equation.
Example: Zn + Cu²⁺ → Zn²⁺ + Cu
Zinc (Zn) loses two electrons to become Zn²⁺ (oxidation), and copper(II) ion (Cu²⁺) gains two electrons to become Cu (reduction). The electron transfer is clearly visible, confirming this as a redox reaction.
3. Recognizing Common Redox Reaction Types
Familiarizing yourself with common types of redox reactions can aid rapid identification. These include:
- Combustion reactions: Reactions with oxygen, often producing heat and light. (e.g., CH₄ + 2O₂ → CO₂ + 2H₂O) Carbon is oxidized, and oxygen is reduced.
- Corrosion: The oxidation of metals in the presence of oxygen and water. (e.g., 4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃) Iron is oxidized.
- Single displacement reactions: One element replaces another in a compound. (e.g., Zn + 2HCl → ZnCl₂ + H₂) Zinc is oxidized, and hydrogen is reduced.
- Disproportionation reactions: A single element undergoes both oxidation and reduction. (e.g., 2Cu⁺ → Cu²⁺ + Cu) Copper(I) is both oxidized and reduced.
4. Using Half-Reactions
A powerful technique to analyze redox reactions is to break them down into two half-reactions: one for oxidation and one for reduction. This clearly highlights the electron transfer.
Example: Let's revisit the reaction: Zn + Cu²⁺ → Zn²⁺ + Cu
- Oxidation half-reaction: Zn → Zn²⁺ + 2e⁻
- Reduction half-reaction: Cu²⁺ + 2e⁻ → Cu
Balancing the number of electrons in both half-reactions ensures that the overall reaction is balanced electrically.
Common Mistakes to Avoid When Identifying Redox Reactions
Even with a solid understanding of the principles, certain pitfalls can lead to misidentification.
- Ignoring spectator ions: Spectator ions do not participate in the redox process. Focus solely on the species that undergo changes in oxidation states.
- Incorrect oxidation state assignment: This is a major source of error. Carefully follow the rules for assigning oxidation states to avoid mistakes.
- Confusing acid-base reactions with redox reactions: Acid-base reactions involve proton transfer, not electron transfer. Do not confuse the two.
- Overlooking disproportionation reactions: The simultaneous oxidation and reduction of the same element might be easily missed if not specifically looked for.
Advanced Techniques and Applications
While the methods described above cover most cases, more advanced techniques exist for complex scenarios. These include:
- Electrochemical methods: Using techniques like potentiometry or voltammetry to measure the change in electrode potential during a reaction.
- Spectroscopic methods: Utilizing techniques like UV-Vis, or NMR spectroscopy to monitor the changes in the electronic or nuclear environment of the atoms involved in the redox process.
Conclusion: Mastering Redox Reaction Identification
Identifying redox reactions is a fundamental skill in chemistry. By systematically applying the methods discussed – analyzing oxidation state changes, observing electron transfer, recognizing reaction types, and utilizing half-reactions – you can confidently determine whether a given reaction involves redox processes. Remember to practice, and with consistent application, you will master this crucial aspect of chemical understanding. This expertise will serve you well in various fields, from environmental chemistry to materials science and beyond. The ability to identify redox reactions is a cornerstone of understanding many crucial processes in our world.
Latest Posts
Latest Posts
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
-
What Are The Five Evaluation Criteria
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Can You Identify A Redox Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
