How Do You Balance A Nuclear Equation
Muz Play
Mar 31, 2025 · 5 min read
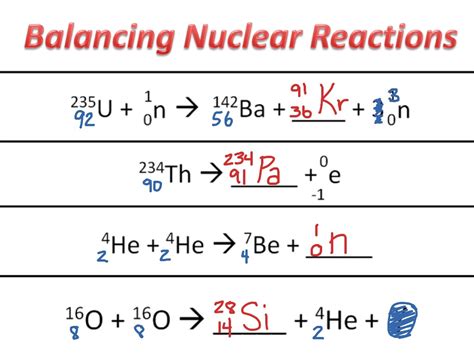
Table of Contents
How to Balance a Nuclear Equation: A Comprehensive Guide
Balancing nuclear equations might seem daunting at first, but with a systematic approach and understanding of the fundamental principles, it becomes a manageable task. This comprehensive guide will walk you through the process, explaining the underlying concepts and providing numerous examples to solidify your understanding. We'll cover everything from basic definitions to advanced techniques, ensuring you gain a thorough grasp of nuclear equation balancing.
Understanding the Fundamentals: Key Concepts
Before diving into the mechanics of balancing, let's establish a strong foundation by defining key terms and principles.
Nuclear Reactions vs. Chemical Reactions:
Unlike chemical reactions that involve the rearrangement of electrons, nuclear reactions involve changes in the nucleus of an atom. These changes can result in the emission of particles (like alpha, beta, or gamma radiation) or the transformation of one element into another. This fundamental difference significantly impacts how we approach balancing.
Key Players: Protons, Neutrons, and Nucleons:
- Protons (p): Positively charged particles found in the nucleus. The number of protons defines the element (atomic number, Z).
- Neutrons (n): Neutral particles also found in the nucleus. The number of neutrons contributes to the isotope's mass number (A).
- Nucleons: The collective term for protons and neutrons. The mass number (A) represents the total number of nucleons in the nucleus.
Isotopes and Isobars:
- Isotopes: Atoms of the same element (same number of protons) but with different numbers of neutrons (different mass numbers). For example, ¹²C and ¹⁴C are isotopes of carbon.
- Isobars: Atoms of different elements with the same mass number (same total number of nucleons). For example, ¹⁴C and ¹⁴N are isobars.
Conservation Laws in Nuclear Reactions:
Two crucial conservation laws govern nuclear reactions:
- Conservation of Mass Number (A): The total mass number of the reactants must equal the total mass number of the products.
- Conservation of Atomic Number (Z): The total atomic number of the reactants must equal the total atomic number of the products.
Balancing Nuclear Equations: A Step-by-Step Approach
Balancing a nuclear equation involves ensuring both the mass number (A) and the atomic number (Z) are conserved on both sides of the equation. Here's a systematic approach:
-
Identify the Reactants and Products: Carefully examine the given nuclear equation and identify all reactants (on the left side) and products (on the right side).
-
Determine the Unknown: Often, one of the reactants or products will be unknown. This is what you need to find.
-
Apply Conservation Laws: Use the conservation of mass number (A) and atomic number (Z) to set up two equations.
-
Solve for the Unknown: Solve the two equations simultaneously to determine the atomic number (Z) and mass number (A) of the unknown particle.
-
Identify the Unknown Particle: Using the atomic number (Z) you determined, identify the element represented by that atomic number.
-
Verify the Balance: Check that the mass number (A) and atomic number (Z) are balanced on both sides of the equation.
Examples of Balancing Nuclear Equations
Let's illustrate the process with various examples, progressing in complexity:
Example 1: Alpha Decay
Uranium-238 undergoes alpha decay. Write the balanced nuclear equation.
Step 1: Identify Reactants and Products
Reactant: ²³⁸U Product: α-particle (⁴He) and an unknown element (X)
Step 2: Write the Unbalanced Equation
²³⁸₉₂U → ⁴₂He + ˣᵧX
Step 3: Apply Conservation Laws
Conservation of mass number (A): 238 = 4 + A => A = 234 Conservation of atomic number (Z): 92 = 2 + Z => Z = 90
Step 4: Identify the Unknown Particle
Element with Z = 90 is Thorium (Th)
Step 5: Write the Balanced Equation
²³⁸₉₂U → ⁴₂He + ²³⁴₉₀Th
Example 2: Beta Decay
Carbon-14 undergoes beta decay. Write the balanced nuclear equation.
Step 1: Identify Reactants and Products
Reactant: ¹⁴C Products: β-particle (⁰₋₁e) and an unknown element (X)
Step 2: Write the Unbalanced Equation
¹⁴₆C → ⁰₋₁e + ˣᵧX
Step 3: Apply Conservation Laws
Conservation of mass number (A): 14 = 0 + A => A = 14 Conservation of atomic number (Z): 6 = -1 + Z => Z = 7
Step 4: Identify the Unknown Particle
Element with Z = 7 is Nitrogen (N)
Step 5: Write the Balanced Equation
¹⁴₆C → ⁰₋₁e + ¹⁴₇N
Example 3: Neutron Capture
Silver-107 captures a neutron. Write the balanced nuclear equation.
Step 1: Identify Reactants and Products
Reactants: ¹⁰⁷₄₇Ag and ¹₀n Product: An unknown element (X)
Step 2: Write the Unbalanced Equation
¹⁰⁷₄₇Ag + ¹₀n → ˣᵧX
Step 3: Apply Conservation Laws
Conservation of mass number (A): 107 + 1 = A => A = 108 Conservation of atomic number (Z): 47 + 0 = Z => Z = 47
Step 4: Identify the Unknown Particle
Element with Z = 47 is Silver (Ag)
Step 5: Write the Balanced Equation
¹⁰⁷₄₇Ag + ¹₀n → ¹⁰⁸₄₇Ag
Example 4: More Complex Reactions
Consider the reaction: ²³⁵₉₂U + ¹₀n → ¹⁴¹₅₆Ba + ⁹²₃₆Kr + x¹₀n
Here, we need to determine the number of neutrons (x) produced.
Step 1: Identify Reactants and Products
Reactants: ²³⁵₉₂U and ¹₀n Products: ¹⁴¹₅₆Ba, ⁹²₃₆Kr, and x¹₀n
Step 2: Apply Conservation Laws
Conservation of mass number (A): 235 + 1 = 141 + 92 + x(1) => x = 3 Conservation of atomic number (Z): 92 + 0 = 56 + 36 + x(0) => Z is balanced, confirming the solution
Step 3: Write the Balanced Equation
²³⁵₉₂U + ¹₀n → ¹⁴¹₅₆Ba + ⁹²₃₆Kr + 3¹₀n
Advanced Concepts and Challenges
While the examples above demonstrate the basic principles, more complex nuclear reactions might involve:
- Multiple Products: Reactions yielding more than two products require careful consideration of all conservation laws.
- Unknown Particles: Determining the identity of unknown particles might involve analyzing the decay scheme or using other experimental data.
- Nuclear Fission and Fusion: These complex processes involve significant energy changes and require a deeper understanding of nuclear physics.
Conclusion: Mastering Nuclear Equation Balancing
Balancing nuclear equations is a fundamental skill in nuclear chemistry and physics. By consistently applying the conservation laws of mass number and atomic number, you can solve a wide range of problems. Remember to approach each problem systematically, identifying the unknowns and carefully checking your work. With practice and a clear understanding of the underlying principles, balancing nuclear equations will become second nature. This detailed guide, with its step-by-step approach and multiple examples, provides a robust foundation for mastering this essential skill. Continue practicing, and you will confidently tackle even the most challenging nuclear equations.
Latest Posts
Latest Posts
-
Where Are Nonmetals Located In The Periodic Table
Apr 02, 2025
-
Write An Equation Any Form For The Quadratic Graphed Below
Apr 02, 2025
-
What Is The Chromosomal Basis Of Inheritance
Apr 02, 2025
-
Two Different Ionic Compounds Each Contain
Apr 02, 2025
-
Part Ii Equilibria Involving Sparingly Soluble Salts
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Do You Balance A Nuclear Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
