How Does A Buffer Solution Resist Change In Ph
Muz Play
Mar 25, 2025 · 7 min read
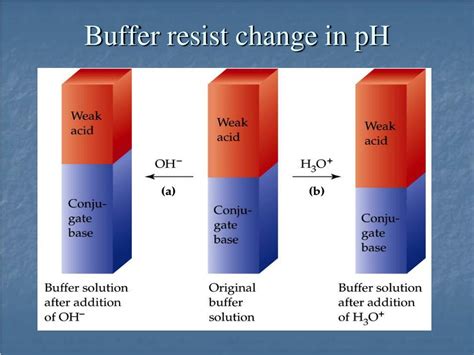
Table of Contents
How Does a Buffer Solution Resist Change in pH?
Maintaining a stable pH is crucial in numerous chemical and biological processes. Fluctuations in pH can dramatically alter reaction rates, enzyme activity, and overall system stability. This is where buffer solutions step in, acting as pH guardians to minimize the impact of added acids or bases. Understanding how these solutions achieve this remarkable feat is key to appreciating their importance in various fields, from chemistry labs to biological systems.
What is a Buffer Solution?
A buffer solution, or simply a buffer, is an aqueous solution that resists changes in pH upon the addition of small amounts of acid or base. It's a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid) in roughly equal concentrations. This unique composition is the key to its pH-buffering capabilities.
The Key Components: Weak Acids and Conjugate Bases
The magic behind a buffer's functionality lies in the equilibrium between the weak acid and its conjugate base. A weak acid is an acid that only partially dissociates in water, meaning it doesn't completely break down into its ions. Its conjugate base is the species formed when the weak acid donates a proton (H⁺). This equilibrium is dynamic; the weak acid and its conjugate base are constantly interconverting.
Consider the generic weak acid, HA:
HA ⇌ H⁺ + A⁻
where:
- HA represents the weak acid
- H⁺ represents the hydrogen ion (proton)
- A⁻ represents the conjugate base
The equilibrium is governed by the acid dissociation constant, Ka, which is a measure of the acid's strength. A lower Ka value indicates a weaker acid.
The Mechanism of pH Resistance
The ability of a buffer to resist pH changes stems from its ability to neutralize both added acids and bases. Let's examine this mechanism in detail:
Neutralizing Added Acids
When a strong acid, such as HCl, is added to a buffer solution, the added H⁺ ions are consumed by the conjugate base (A⁻) present in the buffer:
H⁺ + A⁻ ⇌ HA
This reaction shifts the equilibrium of the buffer system to the left, favoring the formation of the weak acid (HA). The increase in H⁺ concentration is minimized, preventing a significant drop in pH. Because the weak acid only partially dissociates, the impact on the overall pH is far less than if the acid were added to pure water.
Neutralizing Added Bases
Conversely, when a strong base, such as NaOH, is added to the buffer, the added hydroxide ions (OH⁻) react with the weak acid (HA):
OH⁻ + HA ⇌ H₂O + A⁻
This reaction consumes the OH⁻ ions and shifts the equilibrium to the right, increasing the concentration of the conjugate base (A⁻). Again, the change in pH is relatively small because the weak acid is only partially dissociated. The buffer's capacity to neutralize the added base prevents a significant pH increase.
The Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a crucial tool for understanding and calculating the pH of a buffer solution. It directly relates the pH of a buffer to the pKa of the weak acid and the ratio of the concentrations of the conjugate base and weak acid:
pH = pKa + log([A⁻]/[HA])
where:
- pH is the pH of the buffer solution
- pKa is the negative logarithm of the acid dissociation constant (Ka)
- [A⁻] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
This equation highlights the importance of the ratio of [A⁻]/[HA]. When this ratio is close to 1 (meaning the concentrations of the weak acid and conjugate base are roughly equal), the pH of the buffer is approximately equal to the pKa of the weak acid. This is the most effective buffering region.
Buffer Capacity and Effective Range
A buffer's effectiveness isn't unlimited. It has a buffer capacity, which is the amount of acid or base that can be added before the pH changes significantly. The buffer capacity is highest when the concentrations of the weak acid and its conjugate base are approximately equal. As more acid or base is added, the buffer capacity is gradually depleted, and the pH change becomes more pronounced.
The effective range of a buffer is the pH range over which it effectively resists changes in pH. This range is typically within ±1 pH unit of the pKa of the weak acid. Outside this range, the buffer's ability to resist pH changes diminishes significantly.
Examples of Buffer Solutions
Buffer solutions are ubiquitous in various applications. Here are a few notable examples:
Biological Buffers:
- Phosphate buffer: Commonly used in biological systems due to its non-toxicity and compatibility with biological molecules. It's effective within the physiological pH range (around 7.0-7.4).
- Bicarbonate buffer: Plays a vital role in maintaining the pH of blood. It's a crucial component of the body's acid-base balance system.
- Tris buffer: Frequently employed in biochemistry and molecular biology experiments, providing a stable pH environment for sensitive reactions.
Chemical Buffers:
- Acetate buffer: Used in analytical chemistry and industrial processes requiring a stable pH environment.
- Citrate buffer: Frequently used in food and beverage applications, as well as in some chemical processes.
Applications of Buffer Solutions
The ability of buffer solutions to maintain a stable pH makes them indispensable in a wide array of applications:
Maintaining Stable pH in Chemical Reactions:
Many chemical reactions are highly sensitive to pH changes. Buffer solutions ensure that the reaction proceeds under optimal conditions, leading to higher yields and improved product quality. This is particularly crucial in enzyme-catalyzed reactions, where enzyme activity is strongly dependent on pH.
Maintaining Stable pH in Biological Systems:
Buffer solutions are vital in maintaining the pH of biological systems. The human body, for instance, relies heavily on buffer systems to regulate blood pH and maintain cellular function. Slight deviations from the optimal pH range can have severe consequences.
Analytical Chemistry:
Buffer solutions are essential in various analytical techniques, such as titrations, where a stable pH is needed for accurate measurements. They're also used in electrochemical measurements and chromatographic separations.
Medicine and Pharmaceuticals:
Buffer solutions are used extensively in pharmaceutical formulations to stabilize drugs, ensuring their effectiveness and preventing degradation. They are also used in intravenous fluids to maintain the correct pH for safe administration.
Environmental Monitoring:
Buffer solutions are used in environmental monitoring to maintain consistent pH conditions during water quality analysis. Accurate measurements of pH are essential for assessing water quality and environmental impact.
Factors Affecting Buffer Effectiveness
Several factors influence a buffer solution's effectiveness:
-
Concentration of buffer components: A higher concentration of buffer components leads to a higher buffer capacity. However, very high concentrations can introduce other issues.
-
Ratio of weak acid to conjugate base: A ratio close to 1 provides the highest buffer capacity.
-
Temperature: Temperature changes can alter the equilibrium between the weak acid and its conjugate base, affecting the buffer's pH.
-
Ionic strength: The presence of other ions in the solution can affect the activity coefficients of the buffer components, influencing the buffer's effectiveness.
Beyond the Basics: More Complex Buffer Systems
While the simple weak acid/conjugate base system is the foundation of buffering, more complex buffer systems exist, often involving mixtures of multiple weak acids and their conjugate bases to achieve broader effective ranges or higher buffer capacities. These systems are crucial in sophisticated applications like biological systems and industrial processes demanding precise pH control across wide ranges.
Conclusion
Buffer solutions are indispensable tools for maintaining a stable pH in a wide variety of applications. Their ability to resist changes in pH, primarily due to the equilibrium between a weak acid and its conjugate base, is critical in many chemical, biological, and industrial processes. Understanding the principles behind buffer solutions, including the Henderson-Hasselbalch equation and the factors influencing buffer capacity and effective range, is essential for their effective utilization. The applications of buffer solutions are vast, ranging from maintaining the pH of our blood to controlling chemical reactions and ensuring the quality of pharmaceutical products. As we continue to develop more sophisticated technologies and applications, the importance of buffer solutions will only continue to grow.
Latest Posts
Latest Posts
-
Label The Features Of The Thoracic Cage
Mar 26, 2025
-
Kinetic And Potential Energy Of A Pendulum
Mar 26, 2025
-
Is Milk A Homogeneous Mixture Or Heterogeneous
Mar 26, 2025
-
Periodic Table With Metals Nonmetals And Metalloids
Mar 26, 2025
-
How To Find A Collision Force
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Does A Buffer Solution Resist Change In Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
