How Does Atomic Radius Change Across A Period
Muz Play
Mar 23, 2025 · 5 min read
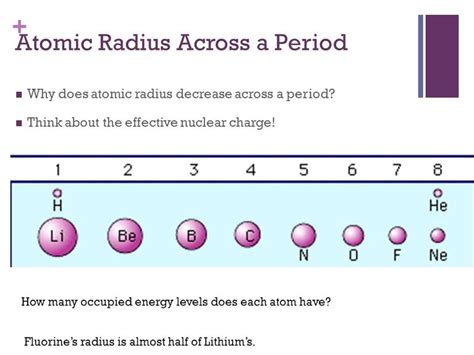
Table of Contents
How Does Atomic Radius Change Across a Period? A Comprehensive Guide
Understanding the periodic trends of elements is fundamental to grasping the principles of chemistry. One such crucial trend is the change in atomic radius across a period (horizontal row) of the periodic table. This article delves deep into this phenomenon, explaining the underlying reasons, exceptions, and its implications in various chemical properties.
What is Atomic Radius?
Before exploring the trend, it's essential to define atomic radius. It's not a directly measurable quantity like, say, the diameter of a marble. Instead, it represents the average distance between the nucleus and the outermost electron in an atom. Determining this distance involves complex quantum mechanical calculations and often relies on experimental data from X-ray diffraction or other spectroscopic techniques. The atomic radius is typically expressed in picometers (pm), where 1 pm = 1 × 10⁻¹² meters.
It's crucial to remember that the electron cloud doesn't have a sharp boundary; its density gradually decreases as you move away from the nucleus. Therefore, various methods to define atomic radius lead to slightly different values, but the overall trend remains consistent.
The Trend: Decrease in Atomic Radius Across a Period
The key takeaway is that atomic radius generally decreases from left to right across a period in the periodic table. This seemingly simple statement hides a complex interplay of nuclear charge and electron shielding. Let's break down the reasons:
1. Increasing Nuclear Charge: The Dominant Factor
As you move across a period, the number of protons in the nucleus increases. This leads to a greater positive nuclear charge. This stronger positive charge pulls the electrons closer to the nucleus, effectively shrinking the atom's size. This is the primary driving force behind the decrease in atomic radius.
2. Electron Shielding: The Less Significant Counteractant
Electrons in the same energy level or shell don't completely shield each other from the nuclear charge. This phenomenon is known as incomplete shielding. While electrons in inner shells shield outer electrons somewhat from the nuclear attraction, the effect is not perfect. The increased nuclear charge outweighs the effect of additional electrons added in the same shell.
3. Effective Nuclear Charge: The Net Effect
The net effect of increased nuclear charge and imperfect shielding is an increase in the effective nuclear charge. This represents the positive charge experienced by the outermost electrons. A higher effective nuclear charge leads to a stronger attraction between the nucleus and the outermost electrons, resulting in a smaller atomic radius.
Visualizing the Trend
Imagine a group of increasingly positively charged balloons (nucleus) attracting a single negatively charged balloon (outermost electron). As you add more positive charge (protons), the negatively charged balloon will be drawn closer, reducing the overall distance. This analogy isn't perfect, but it visually illustrates the core concept.
Exceptions to the General Trend
While the decrease in atomic radius across a period is a general trend, there are a few exceptions, primarily involving electron configurations and specific elements:
-
Transition Metals: The decrease in atomic radius across the transition metal series is less pronounced compared to other periods. This is because the added electrons are filling inner d orbitals, which shield the outer s electrons somewhat more effectively than electrons added to p orbitals.
-
Lanthanides and Actinides: The f-block elements show a similar trend of smaller than expected decrease in atomic radius due to the efficient shielding of the f electrons.
-
Slight Increases: You might find some apparently anomalous slight increases in atomic radius. These are often associated with electron-electron repulsion in sub-shells. When a new subshell is started (e.g., a p subshell after a filled s subshell), the electron-electron repulsion can slightly counter the increased nuclear attraction. These anomalies are smaller in magnitude than the overall trend.
Implications of Atomic Radius
The change in atomic radius across a period has profound implications for various chemical properties:
1. Ionization Energy:
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. As the atomic radius decreases, the outermost electron is held more tightly by the nucleus, requiring more energy for removal.
2. Electronegativity:
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. The smaller atomic radius means the nucleus can exert a stronger pull on shared electrons in a bond.
3. Electron Affinity:
Electron affinity, the energy change associated with gaining an electron, generally shows a complex trend. While it tends to increase, there are exceptions due to electron-electron repulsion and the stability of half-filled and completely filled subshells.
4. Metallic Character:
*Metallic character, the tendency of an element to lose electrons and form positive ions, generally decreases across a period. As the atomic radius decreases and ionization energy increases, it becomes more difficult for atoms to lose electrons.
5. Reactivity:
The reactivity of elements is closely tied to atomic radius and its implications on ionization energy and electronegativity. Elements on the left side of a period (with larger atomic radii and lower ionization energies) are generally more reactive than elements on the right side.
Advanced Considerations: Quantum Mechanical Effects
The simple model presented above provides a good understanding of the general trend, but a complete description requires considering the complex quantum mechanical behavior of electrons. Factors such as electron-electron repulsion, orbital penetration, and relativistic effects play minor but non-negligible roles in the subtle variations observed.
Conclusion: A Fundamental Periodic Trend
The decrease in atomic radius across a period is a fundamental periodic trend directly influencing numerous crucial chemical properties. Understanding this trend is essential for predicting the behavior of elements and their compounds. While the simplified model provides a strong framework, remember that finer details involve complex quantum mechanical phenomena. This comprehensive overview aims to provide both a foundational understanding and a glimpse into the nuances of this important chemical concept. Further research into specific elements and periods will reveal even more intricate details. The interplay between nuclear charge, electron shielding, and electron configurations gives rise to a captivating picture of how atomic structure dictates chemical behavior.
Latest Posts
Latest Posts
-
Example Of A Formal Analysis Of Art
Mar 25, 2025
-
The Second Formulation Of The Categorical Imperative
Mar 25, 2025
-
Elements That Are Gaseous At Room Temperature
Mar 25, 2025
-
What Happens When Acids And Bases Are Mixed
Mar 25, 2025
-
What Is An Intensive Property Of A Substance
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Does Atomic Radius Change Across A Period . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
