How Does Atomic Radius Increase Across The Periodic Table
Muz Play
Mar 22, 2025 · 6 min read
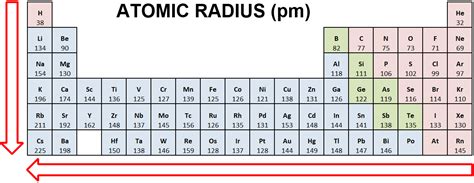
Table of Contents
How Does Atomic Radius Increase Across the Periodic Table?
Understanding atomic radius trends across the periodic table is fundamental to grasping the behavior of elements and their chemical properties. This seemingly simple concept underpins a wealth of chemical phenomena, from reactivity to the formation of compounds. This comprehensive guide will delve deep into the factors influencing atomic radius, examining trends across periods and down groups, and exploring exceptions to these general rules.
Defining Atomic Radius: A Matter of Measurement
Before we explore the trends, it's crucial to precisely define atomic radius. Unfortunately, there's no single, universally accepted definition. The problem stems from the nature of atoms themselves – they don't have sharply defined boundaries. Electrons are not confined to fixed orbits; instead, they exist in a probability cloud surrounding the nucleus.
Therefore, "atomic radius" is usually operationally defined based on the context. Several methods exist for estimating atomic radius, including:
- Metallic radius: Half the distance between the nuclei of two adjacent atoms in a metallic crystal. This is commonly used for metals.
- Covalent radius: Half the distance between the nuclei of two atoms bonded together covalently. This is applicable to nonmetals and metalloids.
- Van der Waals radius: Half the distance between the nuclei of two non-bonded atoms in close proximity. This is used when considering interactions between non-bonded atoms.
These different radii measurements often yield slightly different values for the same atom, but the overall trends across the periodic table remain largely consistent. For the purposes of this discussion, we'll focus on the general trends observed regardless of the specific measurement method.
Trends Across a Period (Left to Right): The Shrinking Atom
As we move across a period from left to right in the periodic table, the atomic radius generally decreases. This seemingly counterintuitive trend arises from the interplay of two key factors:
1. Increasing Nuclear Charge: The Strong Pull
The number of protons in the nucleus (the atomic number) increases as we move across a period. This leads to a stronger positive charge in the nucleus, which exerts a greater attractive force on the electrons.
2. Shielding Effect: A Limited Counteract
While the nuclear charge is increasing, the electrons added are all entering the same principal energy level (shell). The inner electrons shield, or screen, the outer electrons from the full effect of the increased nuclear charge. However, this shielding effect is not completely effective.
The net result is that the increased nuclear charge outweighs the increased shielding, causing a stronger pull on the outer electrons. This pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
In essence: The increasing nuclear charge dominates the shielding effect, causing a decrease in atomic radius across a period.
Trends Down a Group (Top to Bottom): The Expanding Atom
In contrast to the trend across a period, the atomic radius generally increases as we move down a group in the periodic table. This increase is primarily due to the addition of a new principal energy level (shell) with each subsequent element in the group.
1. Adding Energy Levels: Expanding the Space
As you go down a group, each element has one more electron shell than the element above it. These new shells are located further from the nucleus, leading to a larger atomic radius.
2. Shielding Effect: Weakening the Pull
The increase in the number of inner electrons leads to a more effective shielding effect. The outermost electrons are shielded from the increasing nuclear charge more effectively, experiencing a weaker net attractive force.
3. Effective Nuclear Charge: A Diluted Force
The effective nuclear charge (the net positive charge experienced by an outer electron) increases down a group, but not as rapidly as the distance of the outer electrons from the nucleus. The additional distance more than compensates for the increase in effective nuclear charge, resulting in a larger atomic radius.
In essence: The addition of a new principal energy level and the increasing shielding effect dominate the increase in nuclear charge, leading to an increase in atomic radius down a group.
Exceptions and Anomalies: Nuances in the Trends
While the general trends of decreasing atomic radius across a period and increasing atomic radius down a group hold true for most elements, there are notable exceptions and anomalies. These exceptions often arise from:
1. Electron-Electron Repulsion: A Competing Force
The electrons within the same shell repel each other. In some cases, particularly in elements with multiple electrons in the same subshell (e.g., p orbitals), this repulsion can slightly counteract the attractive force of the nucleus. This can lead to a slightly larger atomic radius than would be predicted based solely on nuclear charge and shielding.
2. Lanthanide and Actinide Contraction: Shielding and Nuclear Charge Competition
The lanthanides and actinides, positioned in the f-block of the periodic table, exhibit a unique effect known as the lanthanide and actinide contraction. Poor shielding by the f-electrons results in a stronger effective nuclear charge, leading to smaller than expected atomic radii for these elements and subsequent elements in the periods following them. This effect influences the atomic radii of the elements in the d-block (transition metals).
3. Relativistic Effects: The Speed of Light and Atomic Size
For very heavy elements, relativistic effects start to play a significant role. Electrons in the inner shells move at speeds approaching the speed of light. According to Einstein's theory of special relativity, at such high speeds, the mass of the electron increases. This increased mass causes the inner electrons to move closer to the nucleus, reducing their shielding effectiveness and thus impacting the atomic radius. This effect is particularly noticeable in the heavier elements, causing deviations from the expected trends.
Applications and Significance: Atomic Radius in Action
Understanding atomic radius trends is crucial in various fields of chemistry and related disciplines:
-
Chemical Reactivity: Atomic radius significantly influences an element's reactivity. Smaller atoms generally have higher ionization energies and electronegativities, making them less likely to lose electrons and more likely to attract electrons from other atoms. Conversely, larger atoms have lower ionization energies and electronegativities, making them more likely to lose electrons and less likely to attract them.
-
Ionic Radii and Compound Formation: The size of ions (charged atoms) is directly related to their atomic radii. The formation of ionic compounds depends on the relative sizes of the cation (positive ion) and anion (negative ion).
-
Crystal Structures and Properties: The arrangement of atoms in a crystal lattice is determined by the size of the atoms involved. Atomic radius affects the density, melting point, and other physical properties of solid materials.
-
Catalysis: The size and shape of atoms in catalysts are critical to their function. The ability of a catalyst to bind to reactants and facilitate a reaction depends on the atomic radii of the atoms involved.
Conclusion: A Comprehensive View of Atomic Radius Trends
Atomic radius is not a static property; it's a dynamic aspect of atomic structure significantly influenced by nuclear charge, shielding effects, electron-electron repulsion, and, in the case of heavier elements, relativistic effects. While general trends exist, the periodic table reveals subtle deviations that highlight the complexities of atomic structure and their influence on elemental properties. A thorough understanding of these trends is essential for grasping chemical behavior and predicting the properties of elements and their compounds. The study of atomic radius serves as a cornerstone for comprehending the fascinating and multifaceted world of chemistry.
Latest Posts
Latest Posts
-
Addition And Subtraction Properties Of Equality
Mar 23, 2025
-
How To Calculate Kb When Only Given Ka
Mar 23, 2025
-
Steps For Conducting A Presumptive Blood Test
Mar 23, 2025
-
The Plasma Membrane Of A Muscle Fiber Is Called The
Mar 23, 2025
-
Relationship Between Voltage And Electric Field
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Does Atomic Radius Increase Across The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
