How To Calculate Kb When Only Given Ka
Muz Play
Mar 23, 2025 · 5 min read
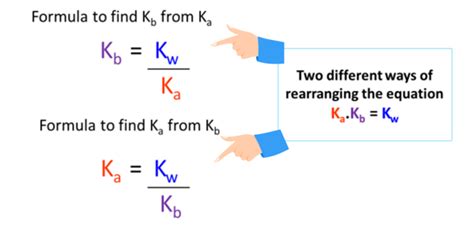
Table of Contents
How to Calculate Kb When Only Given Ka
Determining the base dissociation constant (Kb) might seem daunting when you only have the acid dissociation constant (Ka) at your disposal. However, with a solid understanding of the relationship between acids and bases, and the application of a simple formula, the calculation becomes straightforward. This comprehensive guide will walk you through the process, exploring the underlying concepts, providing step-by-step instructions, and offering practical examples to solidify your understanding.
Understanding Ka and Kb: The Acid-Base Duo
Before diving into the calculations, let's establish a firm grasp of the fundamental concepts of Ka and Kb. These constants are crucial in understanding the strength of acids and bases in aqueous solutions.
-
Ka (Acid Dissociation Constant): This constant quantifies the extent to which an acid dissociates (breaks apart) into its conjugate base and a proton (H⁺) in water. A higher Ka value indicates a stronger acid, meaning it dissociates more readily. The equation for Ka is:
Ka = [H⁺][A⁻] / [HA]
where:
- [H⁺] represents the concentration of hydrogen ions
- [A⁻] represents the concentration of the conjugate base
- [HA] represents the concentration of the undissociated acid
-
Kb (Base Dissociation Constant): Similarly, Kb quantifies the extent to which a base dissociates into its conjugate acid and a hydroxide ion (OH⁻) in water. A higher Kb value indicates a stronger base. The equation for Kb is:
Kb = [OH⁻][HB⁺] / [B]
where:
- [OH⁻] represents the concentration of hydroxide ions
- [HB⁺] represents the concentration of the conjugate acid
- [B] represents the concentration of the undissociated base
The Interplay Between Ka and Kb: The Water Ion Product
The beauty of acid-base chemistry lies in the interconnectedness of its components. The relationship between Ka and Kb is defined by the ion product of water (Kw). Kw is a constant at a given temperature, commonly taken as 1.0 x 10⁻¹⁴ at 25°C. The relationship is expressed as:
Kw = Ka * Kb
This equation is the key to calculating Kb when only Ka is known. By rearranging the equation, we can solve for Kb:
Kb = Kw / Ka
This simple formula allows us to determine the base dissociation constant of the conjugate base if we know the acid dissociation constant of its conjugate acid. Let's delve into the process with some examples.
Step-by-Step Calculation of Kb from Ka
Let's illustrate the calculation with a step-by-step approach using hypothetical examples.
Example 1: A Weak Acid
Suppose we are given that the Ka of a weak acid, HA, is 2.0 x 10⁻⁵ at 25°C. We want to calculate the Kb of its conjugate base, A⁻.
Step 1: Identify Kw
At 25°C, Kw = 1.0 x 10⁻¹⁴
Step 2: Apply the formula
Kb = Kw / Ka = (1.0 x 10⁻¹⁴) / (2.0 x 10⁻⁵) = 5.0 x 10⁻¹⁰
Therefore, the Kb of the conjugate base, A⁻, is 5.0 x 10⁻¹⁰. This value indicates that the conjugate base is a very weak base.
Example 2: A Stronger Acid
Let's consider a stronger acid with a Ka of 1.8 x 10⁻⁴ at 25°C.
Step 1: Identify Kw
Kw remains 1.0 x 10⁻¹⁴ at 25°C.
Step 2: Apply the formula
Kb = Kw / Ka = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁴) = 5.6 x 10⁻¹¹
In this case, the Kb of the conjugate base is 5.6 x 10⁻¹¹. Notice that even though the acid is stronger (higher Ka), its conjugate base is still relatively weak (lower Kb).
Dealing with Polyprotic Acids
Polyprotic acids can donate more than one proton. Calculating Kb for polyprotic acid systems requires a slightly more nuanced approach. Each dissociation step has its own Ka value. You would use the appropriate Ka for the relevant conjugate base.
For example, a diprotic acid (H₂A) has two Ka values: Ka1 and Ka2. To calculate the Kb for the conjugate base HA⁻, you would use Ka2 in the equation Kb = Kw / Ka2. This is because HA⁻ is the conjugate base formed after the second proton is donated.
Practical Applications and Significance
The ability to calculate Kb from Ka has numerous applications in chemistry and related fields. Here are some key areas:
-
Buffer Solutions: Understanding both Ka and Kb is essential in designing buffer solutions, which resist changes in pH. The Henderson-Hasselbalch equation utilizes both Ka and Kb to predict the pH of buffer systems.
-
Titration Curves: Calculations involving Ka and Kb are crucial in analyzing and predicting the shapes of titration curves, which provide critical information about acid-base reactions.
-
Solubility Equilibria: Understanding the relationship between Ka and Kb can aid in solving problems related to the solubility of sparingly soluble salts in water. Many solubility problems require consideration of acid-base equilibria.
Advanced Considerations and Limitations
While the formula Kb = Kw / Ka is powerful and widely applicable, it's important to acknowledge certain limitations:
-
Temperature Dependence: Kw is temperature-dependent. The value of 1.0 x 10⁻¹⁴ is only valid at 25°C. For calculations at different temperatures, the appropriate Kw value must be used.
-
Ionic Strength: The presence of significant concentrations of ions in the solution can affect the activity of the acid and base species, thus influencing the values of Ka and Kb. Activity coefficients might need to be considered for more accurate calculations in solutions with high ionic strength.
-
Non-ideal Behavior: At high concentrations, the behavior of acids and bases may deviate from the ideal behavior assumed in the simple equations. More complex models may be required for accurate calculations under non-ideal conditions.
Conclusion: Mastering the Ka-Kb Relationship
Calculating Kb from Ka is a fundamental skill in acid-base chemistry. Understanding the relationship between these constants, defined by the ion product of water, empowers you to analyze and predict the behavior of acids and bases in aqueous solutions. By mastering the simple formula and applying it with careful consideration of the limitations, you can gain a deeper understanding of acid-base equilibria and their widespread applications in various chemical contexts. Remember to always account for the temperature and consider the potential impact of ionic strength on the accuracy of your calculations. With practice, you will develop confidence in handling these calculations and gain a valuable tool for problem-solving in chemistry.
Latest Posts
Latest Posts
-
Fcc Unit Cell Number Of Atoms
Mar 25, 2025
-
How Is Temperature Related To Kinetic Energy
Mar 25, 2025
-
Which Element Has The Largest Ionization Energy
Mar 25, 2025
-
What Is The Oxidation Number For Sulfur
Mar 25, 2025
-
Adp Atp And Cellular Respiration Practice Questions
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Kb When Only Given Ka . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
