What Is The Oxidation Number For Sulfur
Muz Play
Mar 25, 2025 · 6 min read
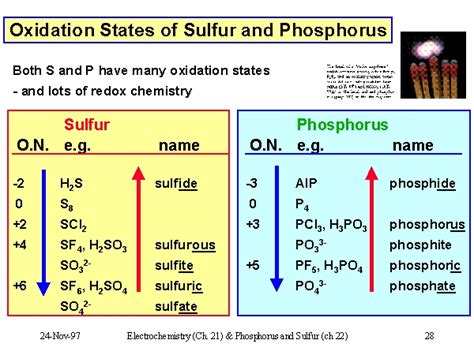
Table of Contents
What is the Oxidation Number for Sulfur? A Comprehensive Guide
Sulfur, a vibrant yellow nonmetal, plays a crucial role in various chemical processes. Understanding its oxidation number is fundamental to comprehending its diverse chemical behavior and its participation in redox reactions. This comprehensive guide delves deep into the oxidation number of sulfur, exploring its variable nature, the rules for determining it, and its significance in different chemical contexts.
Understanding Oxidation Numbers
Before we dive into the specifics of sulfur's oxidation number, let's establish a clear understanding of the concept itself. The oxidation number, also known as the oxidation state, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool in chemistry for balancing redox reactions, predicting the reactivity of elements, and understanding the bonding characteristics of compounds.
It's important to note that oxidation numbers are assigned based on a set of rules, and they don't necessarily reflect the true charge on an atom in a molecule, especially in covalent compounds where electron sharing is prevalent. However, they are incredibly useful for bookkeeping electrons in chemical reactions.
Rules for Assigning Oxidation Numbers
Several rules govern the assignment of oxidation numbers. These rules, applied systematically, help us determine the oxidation state of each atom in a molecule or ion. Here are the key rules:
-
The oxidation number of an atom in its elemental form is always zero. For example, the oxidation number of S in S₈ (sulfur in its elemental state) is 0.
-
The oxidation number of a monatomic ion is equal to its charge. For instance, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
The oxidation number of hydrogen is typically +1. However, in metal hydrides (e.g., NaH), its oxidation number is -1.
-
The oxidation number of oxygen is typically -2. Exceptions exist, such as in peroxides (e.g., H₂O₂) where it's -1, and in superoxides (e.g., KO₂) where it's -1/2.
-
The oxidation number of fluorine is always -1.
-
The sum of oxidation numbers of all atoms in a neutral molecule is zero.
-
The sum of oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
The Variable Oxidation States of Sulfur
Unlike some elements that exhibit a limited range of oxidation states, sulfur demonstrates remarkable versatility, showcasing a wide spectrum of oxidation numbers. This variability stems from sulfur's electronic configuration, allowing it to readily gain or lose electrons to achieve stability. Sulfur's ability to form multiple bonds further contributes to this diversity.
The most common oxidation states for sulfur are:
-
-2: This is the most common and stable oxidation state for sulfur, found in many sulfides (e.g., H₂S, FeS) where sulfur acts as a chalcogen. In this state, sulfur accepts two electrons to achieve a stable octet configuration.
-
+2: This oxidation state is less common than -2 but appears in some compounds like sulfur dioxide (SO₂). Here, sulfur has lost two electrons.
-
+4: Found in compounds such as sulfur dioxide (SO₂) and sulfurous acid (H₂SO₃), this oxidation state indicates sulfur's participation in partially oxidized forms.
-
+6: This is the highest and most oxidized state for sulfur, commonly observed in sulfuric acid (H₂SO₄) and sulfates (SO₄²⁻). In this state, sulfur has lost six electrons.
Determining Sulfur's Oxidation Number in Different Compounds
Let's apply the rules to determine the oxidation number of sulfur in a few key examples:
Example 1: Hydrogen Sulfide (H₂S)
- Oxidation number of hydrogen (H): +1 (Rule 3)
- Let the oxidation number of sulfur (S) be 'x'.
- Total charge of the molecule is 0 (Rule 6).
- Therefore, 2(+1) + x = 0, which solves to x = -2.
- The oxidation number of sulfur in H₂S is -2.
Example 2: Sulfur Dioxide (SO₂)
- Oxidation number of oxygen (O): -2 (Rule 4)
- Let the oxidation number of sulfur (S) be 'x'.
- Total charge of the molecule is 0 (Rule 6).
- Therefore, x + 2(-2) = 0, which solves to x = +4.
- The oxidation number of sulfur in SO₂ is +4.
Example 3: Sulfuric Acid (H₂SO₄)
- Oxidation number of hydrogen (H): +1 (Rule 3)
- Oxidation number of oxygen (O): -2 (Rule 4)
- Let the oxidation number of sulfur (S) be 'x'.
- Total charge of the molecule is 0 (Rule 6).
- Therefore, 2(+1) + x + 4(-2) = 0, which solves to x = +6.
- The oxidation number of sulfur in H₂SO₄ is +6.
Example 4: Sulfate Ion (SO₄²⁻)
- Oxidation number of oxygen (O): -2 (Rule 4)
- Let the oxidation number of sulfur (S) be 'x'.
- Total charge of the ion is -2 (Rule 7).
- Therefore, x + 4(-2) = -2, which solves to x = +6.
- The oxidation number of sulfur in SO₄²⁻ is +6.
Significance of Sulfur's Variable Oxidation States
The variable oxidation states of sulfur are responsible for its diverse chemical properties and its involvement in a wide range of chemical reactions. This versatility makes sulfur a crucial element in many natural and industrial processes.
-
Redox Reactions: Sulfur's ability to exist in various oxidation states makes it a key participant in redox reactions, where electron transfer occurs. This is vital in many biological and industrial processes.
-
Environmental Chemistry: Sulfur compounds, particularly sulfur oxides, play significant roles in atmospheric chemistry and contribute to acid rain formation. Understanding the oxidation states helps in analyzing and mitigating these environmental impacts.
-
Industrial Applications: Sulfur and its compounds are extensively used in various industries, including the production of sulfuric acid (a cornerstone of the chemical industry), fertilizers, and pharmaceuticals. The oxidation state of sulfur dictates the chemical properties and reactivity crucial for these applications.
-
Biological Systems: Sulfur is an essential component of many biological molecules, including amino acids (cysteine and methionine) and proteins. The oxidation state of sulfur influences the structure and function of these biomolecules.
Conclusion: A Versatile Element
Sulfur's oxidation number is not a fixed value but a variable that reflects its capacity to participate in various chemical reactions. Its ability to exist in multiple oxidation states, ranging from -2 to +6, highlights its versatility and significance across various fields, from environmental science and industrial applications to biological systems. By understanding the rules for assigning oxidation numbers and the significance of sulfur's variable oxidation states, we gain a more profound understanding of its fundamental role in the chemical world. This comprehensive knowledge is crucial for anyone studying chemistry, environmental science, or related fields. Mastering this concept opens doors to comprehending complex chemical reactions and their real-world implications.
Latest Posts
Latest Posts
-
How To Calculate Freezing Point Of A Solution
Mar 28, 2025
-
What Is The Monomer That Makes Up Dna
Mar 28, 2025
-
Covalent Bonds Are Formed Between Two Non Metals
Mar 28, 2025
-
In General Pathogens Grow Very Slowly
Mar 28, 2025
-
Mixing An Acid And A Base
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation Number For Sulfur . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
