How To Calculate Freezing Point Of A Solution
Muz Play
Mar 28, 2025 · 7 min read
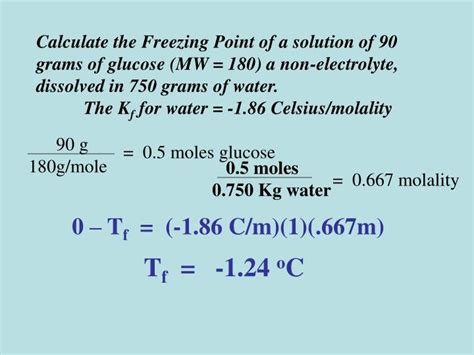
Table of Contents
How to Calculate the Freezing Point of a Solution: A Comprehensive Guide
Determining the freezing point of a solution is crucial in various fields, from chemistry and chemical engineering to environmental science and food technology. Understanding this concept allows for precise control of processes, accurate predictions of behavior, and a deeper understanding of fundamental physical chemistry principles. This comprehensive guide will delve into the methods for calculating the freezing point depression of a solution, covering both theoretical underpinnings and practical applications.
Understanding Freezing Point Depression
The freezing point of a pure solvent is the temperature at which it transitions from a liquid to a solid state. When a solute is added to a solvent, the freezing point of the resulting solution is lower than that of the pure solvent. This phenomenon is known as freezing point depression. It's a colligative property, meaning it depends on the number of solute particles present, not their identity. More solute particles mean a greater decrease in the freezing point.
The Role of Solute Particles
The extent of freezing point depression is directly related to the concentration of solute particles in the solution. This is because the solute particles disrupt the solvent's ability to form a regular crystalline structure during freezing. The solvent molecules need to overcome the interference from solute particles to arrange themselves into the solid state, requiring a lower temperature.
Ideal vs. Non-Ideal Solutions
The calculations presented below assume ideal solutions, where interactions between solute and solvent molecules are negligible. In reality, many solutions deviate from ideality, especially at higher concentrations. For non-ideal solutions, more sophisticated methods, often involving experimental data and activity coefficients, are necessary. This guide will focus on the simpler, ideal solution calculations.
Calculating Freezing Point Depression: The Formula
The freezing point depression (ΔTf) can be calculated using the following formula:
ΔTf = Kf * m * i
Where:
-
ΔTf: The change in freezing point (in °C or K). This is the difference between the freezing point of the pure solvent and the freezing point of the solution. ΔTf is always positive because the freezing point is lowered.
-
Kf: The cryoscopic constant (in °C kg/mol or K kg/mol) of the solvent. This is a characteristic property of the solvent and represents the freezing point depression caused by 1 molal solution of a non-dissociating solute. You'll typically need to look up this value in a reference table.
-
m: The molality of the solution (in mol/kg). Molality is defined as the number of moles of solute per kilogram of solvent. It's crucial to use molality, not molarity (moles of solute per liter of solution), because molality is independent of temperature, unlike molarity.
-
i: The van't Hoff factor. This is a dimensionless factor that accounts for the number of particles a solute dissociates into in solution.
- For non-electrolytes (substances that do not dissociate in solution, like sugar or urea), i = 1.
- For strong electrolytes (substances that completely dissociate in solution, like NaCl or KCl), i is equal to the number of ions formed upon dissociation. For example, NaCl (sodium chloride) has i = 2 (Na⁺ and Cl⁻), while CaCl₂ (calcium chloride) has i = 3 (Ca²⁺ and 2Cl⁻).
- For weak electrolytes (substances that partially dissociate in solution, like acetic acid), i is between 1 and the theoretical number of ions, and its determination is more complex, often requiring experimental data or iterative calculations.
Step-by-Step Calculation Procedure
Let's illustrate the calculation process with a detailed example. Suppose we want to calculate the freezing point of a solution containing 10.0 g of glucose (C₆H₁₂O₆) dissolved in 250 g of water.
Step 1: Determine the molality (m)
-
Find the molar mass of glucose: The molar mass of glucose (C₆H₁₂O₆) is (6 * 12.01 g/mol) + (12 * 1.01 g/mol) + (6 * 16.00 g/mol) = 180.18 g/mol.
-
Calculate the moles of glucose: Moles of glucose = (10.0 g) / (180.18 g/mol) = 0.0555 mol
-
Convert the mass of solvent to kilograms: Mass of water = 250 g = 0.250 kg
-
Calculate the molality: Molality (m) = (0.0555 mol) / (0.250 kg) = 0.222 mol/kg
Step 2: Find the cryoscopic constant (Kf) for the solvent
The cryoscopic constant for water is 1.86 °C kg/mol. This value is readily available in chemical handbooks or online resources.
Step 3: Determine the van't Hoff factor (i)
Glucose is a non-electrolyte, so i = 1.
Step 4: Calculate the freezing point depression (ΔTf)
ΔTf = Kf * m * i = (1.86 °C kg/mol) * (0.222 mol/kg) * (1) = 0.413 °C
Step 5: Calculate the freezing point of the solution
The freezing point of pure water is 0 °C. Therefore, the freezing point of the glucose solution is:
Freezing point of solution = Freezing point of pure water - ΔTf = 0 °C - 0.413 °C = -0.413 °C
Handling Electrolytes: A More Complex Scenario
Let's consider a solution of 5.85 g of NaCl (sodium chloride) dissolved in 500 g of water.
Step 1: Determine the molality (m)
-
Find the molar mass of NaCl: The molar mass of NaCl is 22.99 g/mol + 35.45 g/mol = 58.44 g/mol.
-
Calculate the moles of NaCl: Moles of NaCl = (5.85 g) / (58.44 g/mol) = 0.100 mol
-
Convert the mass of solvent to kilograms: Mass of water = 500 g = 0.500 kg
-
Calculate the molality: Molality (m) = (0.100 mol) / (0.500 kg) = 0.200 mol/kg
Step 2: Find the cryoscopic constant (Kf) for the solvent
Again, Kf for water is 1.86 °C kg/mol.
Step 3: Determine the van't Hoff factor (i)
NaCl is a strong electrolyte, completely dissociating into Na⁺ and Cl⁻ ions. Therefore, i = 2.
Step 4: Calculate the freezing point depression (ΔTf)
ΔTf = Kf * m * i = (1.86 °C kg/mol) * (0.200 mol/kg) * (2) = 0.744 °C
Step 5: Calculate the freezing point of the solution
Freezing point of solution = Freezing point of pure water - ΔTf = 0 °C - 0.744 °C = -0.744 °C
Applications of Freezing Point Depression Calculations
The ability to calculate freezing point depression has wide-ranging applications:
-
Antifreeze: Antifreeze solutions in car radiators utilize the principle of freezing point depression to prevent the coolant from freezing in cold weather. Ethylene glycol is commonly used as a solute to lower the freezing point of water.
-
De-icing: Salts, like NaCl and CaCl₂, are spread on roads and pavements to lower the freezing point of water and prevent ice formation.
-
Food Preservation: Freezing point depression plays a role in food preservation techniques, as the freezing point of food solutions is lowered by the presence of solutes.
-
Chemical Engineering: Accurate determination of freezing points is crucial in various chemical processes, particularly those involving crystallization and separation techniques.
-
Environmental Science: Freezing point measurements are used to analyze water samples and determine the presence and concentration of dissolved substances.
Limitations and Considerations
It's important to acknowledge the limitations of the simple freezing point depression formula:
-
Ideality Assumption: The formula is based on the assumption of ideal solutions. Deviations from ideality can occur, especially at high concentrations, requiring more sophisticated models.
-
Association and Dissociation: The van't Hoff factor (i) can be challenging to determine accurately for weak electrolytes or substances that undergo association or complex formation in solution.
-
Ionic Strength: In solutions with high ionic strength, ion-ion interactions can significantly affect the freezing point, and the simple formula may not be accurate.
-
Solvent Purity: The accuracy of the calculation depends on the purity of the solvent. Impurities can affect the freezing point.
Conclusion
Calculating the freezing point of a solution is a fundamental concept in chemistry with diverse practical applications. This guide has provided a detailed explanation of the underlying principles, a step-by-step calculation procedure, and a discussion of the limitations and considerations. While the simplified formula is useful for many scenarios, it's crucial to remember its assumptions and consider more advanced methods when dealing with non-ideal solutions or complex systems. By understanding these concepts, one can accurately predict and manipulate the freezing points of solutions, leading to advancements in various fields.
Latest Posts
Latest Posts
-
How To Round Sig Figs When Adding
Mar 31, 2025
-
Do Acids Or Bases React With Metals
Mar 31, 2025
-
What Are The Energy Yielding Nutrients
Mar 31, 2025
-
Angle For Bonds Ammonia And Water
Mar 31, 2025
-
Who Created The Law Of Conservation Of Mass
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Freezing Point Of A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
